 I’ve presented on this a couple of times but this is gonna require a few parts, so be patient with me. This is not quite a deep-dive in the SMART EM sense, more of a shallow dive you might do with a snorkel rather than a full on SCUBA.
I’ve presented on this a couple of times but this is gonna require a few parts, so be patient with me. This is not quite a deep-dive in the SMART EM sense, more of a shallow dive you might do with a snorkel rather than a full on SCUBA.
Thrombolysis for stroke is a controversial treatment. SAEM previously had a statement saying
It is the position of the American Academy of Emergency Medicine that objective evidence regarding the efficacy, safety, and applicability of tPA for acute ischemic stroke is insufficient to warrant its classification as standard of care. Until additional evidence clarifies such controversies, physicians are advised to use their discretion when considering its use
This has recently been retracted
Many don’t see it as controversial at all, just an underused treatment that has solid and conclusive proof behind it. The drive to treatment has inspired a big public health initiativ

I don’t mean in this series of posts to discrad the idea that thrombolysis is useful in ischaemic stroke, I just mean to explore the evidence for its use and show that its evidence base makes it a controversial treatment, I would argue it makes it an experimental treatment.
Thrombolysis came into every day use with its role in STEMI; initially with streptokinase and then succeeded by tPA.
The evidence for this involves thousands and thousands of patients in high quality RCTs (GISSI-1 12000; ISIS 2 17000; GUSTO I – 40000)
In addition, in STEMI we have a clearly defined disease process, with a quick and easy to interpret diagnostic test with some important, but usually easily defined mimics.
In contrast, the current use and license for tPA in stroke is based on a single RCT in the mid-90s which was based on 300 people. (I know there were 600 in the trial but I’ll expand on that later).
The more recent discussion (and indeed the AHA level I recommendation) for 3-4.5 hrs is based on the ECASS III trial and I’ll cover that too.
Let me say again, my aim is not to prove to you that tPA for stroke is of no use in stroke, my aim is to make it clear that we are not yet at the stage of being able to say definitive things about its role.
There have so far been 11 published RCTs (that I know of) of the use of thrombolytic therapy for acute ischaemic stroke.
2 of these can be regarded as positive, 9 as negative (the EPITHET trial is a bit unusual in its outcomes and I consider it negative but we’ll cover that). Please take note of that.
Three of the earlier ones concerned the use of streptokinase (ASK, MAST-ITALY, MAST-EUROPE). Two were negative, one was stopped early due to harm. I’m not gonna cover them in this series as no one is actually using strep for stroke these days. I’ll put a link to them in the references.
Let me start with the key trial in this whole thing – NINDS
PATIENTS
First thing to note is that this was actually two trials reported as one.
Part 1 assessed changes in neurologic deficits 24 hours after the onset of stroke as a measure of the activity of t-PA
Part 2, the pivotal study, used four outcome measures representing different aspects of recovery from stroke to assess whether treatment with t-PA resulted in sustained clinical benefit at three months.
Part one wanted to assess whether if you were given tPA or placebo whether you would have a 4 point improvement in your NIHSS at 24 hrs. This is very different from living independently at 3 months; something we really care about.
In both parts the time to treatment was divided into two – 0-90 mins and 91-180 mins. There was a requirement for equal numbers in both the early and late groups. It is extrmely difficult to have a stroke, get to hospital, get seen, get a CT and a decision to get tPA within 90 mins. Most of the people given thrombolysis in real life are in the 91-180 min group. In some of the other trials with a more liberal protocol (up to 6 hrs) the average time is around 4 hrs.
In NINDS the radiographic criteria were a base-line computed tomographic (CT) scan of the brain that showed no evidence of intracranial hemorrhage. Those of you who have read about stroke lysis before wil realise that this is not considered an appropriate CT criteria these days. Indeed I’ve heard a few neuroradiologists and neurologists say that you shouldn’t be giving tPA unless you can see some early signs of infarction on the base-line scan.
OUTCOMES
you should have a primary outcome for your trial. In NINDS they had 4. That’s kind of a cheat. The more you have, the more likely you’re gonna have one turn positive.
they also included the NIHSS as one of their 3 month outcomes which which raises an important point. The NIHSS is a good marker of stroke severity but it’s not necessarily linear. The difference between a 12 and 16 is not necessarily the same as the difference between a 20 and a 24. So if you see a 4 point difference in the NIHSS at 2 hrs or 3 months, that doesn’t mean you have a comparable clinically significant improvement. This is not what the NIHSS had been used for before – the authors even state this in the paper.
They sensibly defined new stroke and bleeding as adverse events but note their definition of symptomatic haemorrhage
A hemorrhage was considered symptomatic if it was not seen on a previous CT scan and there had subsequently been either a suspicion of hemorrhage or any decline in neurologic status
Again, if you’ve been following the literature, you’ll see that this definition varies from study to study
If you died in this trial, all that got recorded was your death and the presumed cause, if you died suddenly from an ICH and you didn’t get a post-mortem (this wasn’t a requirement) then this wasn’t noted.
RESULTS
The first thing is to look at the patients we’re interested in. For brevity I’m only gonna look at part 2; the group that had 3 month outcomes assessed (the only thing we really care about).
(For the record part 1 was negative in its primary outcome.)
If you look at the table in the paper they look reasonably well matched. Mann in the Western Journal of Emergency Medicine put some work in on this and eventually got the full data set from the NINDS investigators and they found some more significant differences in the two groups than is apparent in the table in the NEJM
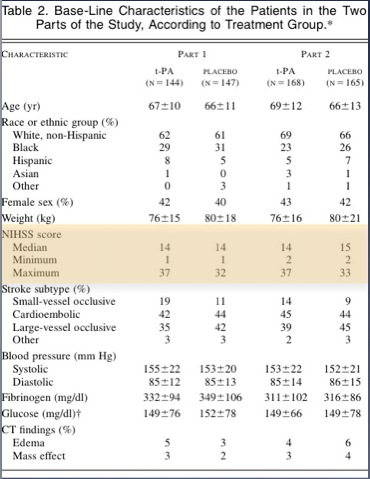
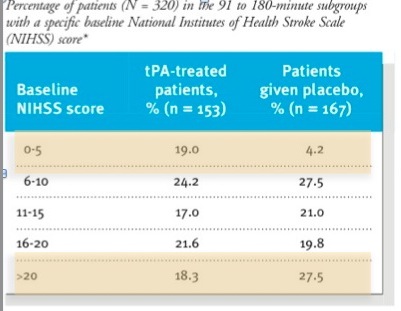
The tables show that patients in the placebo group had more severe strokes.
This doesn’t mean anyone fiddled the trial so that the sicker patients ended up in the placebo group; this variation is easily possible when you have two groups of only 150 each.
Having said that , the tPA group fared better in all 4 outcomes. there was a 12% absolute difference in the Modified Rankin Score (this has become the standard for outcomes in stroke research – a score of 1 or less is considered good outcome)
On the basis of Part 2 of this trial (one group of 150 who got placebo, one group of 150 who got tPA) tPA got FDA approval.
Despite calls for further research, once it got FDA approval, people came out and said it would be unethical to repeat a placebo controlled trial at 0-3 hrs as tPA was “proven” to be helpful.
So much for the NINDS trial.
Next I’ll try and cover some of the other trials arranged by the groups who investigated them.
What’s your take on the NINDS trial and the evidence for thrombolytics in stroke?
References and further reading.
The 11 trials that I know of:
- Randomised controlled trial of streptokinase, aspirin, and combination of both in treatment of acute ischaemic stroke. Multicentre Acute Stroke Trial–Italy (MAST-I) Group. The Lancet 1995 Dec.;346(8989):1509 -1514. PMID: 7491044
- Thrombolytic therapy with streptokinase in acute ischemic stroke. The Multicenter Acute Stroke Trial–Europe Study Group. N Engl J Med 1996 Jul.;335(3):145–150. PMID: 8657211
- Streptokinase for acute ischemic stroke with relationship to time of administration: Australian Streptokinase (ASK) Trial Study Group. JAMA 1996 Sep.;276(12):961–966. PMID: 8805730
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995 Dec.;333(24):1581–1587. PMID: 7477192
- Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995 Oct.;274(13):1017–1025.1. PMID: 7563451
- Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. The Lancet 1998 Oct.;352(9136):1245–1251. PMID: 9788453
- Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008 Sep.;359(13):1317–1329. PMID: 18815396
- Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999 Dec.;282(21):2019–2026. PMID: 10591384
- The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g) : results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke 2000 Apr.;31(4):811–816. PMID 10753980
- Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurology 2009 Feb.;8(2):141–150. PMCID 2730486
- Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurology 2008 Apr.;7(4):299–309.PMID 18296121
- The NNT on this
- Mann J. Efficacy of Tissue Plasminogen Activator (Tpa) for Stroke: Truths about the NINDS study: setting the record straight. Western Journal of Medicine 2002;176(3):192. PMCID: PMC1071714
- Hoffman JR, Schriger DL. A graphic reanalysis of the NINDS Trial. Ann Emerg Med 2009 Sep.;54(3):329–36, 336.e1–35. PMID: 19464756


Fantastic first post on this Andy. I agree with your discomfort about the existing evidence w.r.t. this therapy. The extra issue here is that we need to be absolutely sure of a benefit with this therapy because the patients that die or get ICH get it directly because of the injection we give them. I.e., if we are wrong, we are just killing people. This frequently happens with a direct temporal relationship while they are right under our noses, in our EDs, in our care. I will admit that from observed experience of neurologists lysing patients I referred to them (N=3 –> 2x[ICH+death]) I am perhaps a little coloured on this, but all the more reason I need some serious convincing. First do no harm…looking forward to the follow up posts. D
Cheers Domhnall
you’re right, there’s a whole lot more at stake with lytics for stroke than MI. The symptomatic haemorrhage is about 1% with STEMI and probably 5-8% with stroke. that’s a whole lot more potential for harm.
there are lots of conceptual and systems based difficulties in lytics for stroke too – maybe i’ll cover them when i get through the RCTs! this is gonna be a bit of an epic i think…
Many thanks for this, extremely useful summary. I had not realised that the evidence base for thrombolysis was this sketchy! It does make you wary about how enthusiastically this therapy has been embraced.
Cheers for the comment. The NINDS study is just for starters! I’ve got about 4 more posts on this stuff to come.
Pingback: The LITFL Review 024 - Life in the FastLane Medical Blog
Pingback: Evidence for thrombolytics in stroke – Part 3 | Emergency Medicine Ireland
Pingback: Evidence for thrombolytics in stroke – part 4 | Emergency Medicine Ireland
Pingback: Diabetes, old stroke and tPA « Neurology Journal
Pingback: Scaring the SHO’s or “Can we be sure discharge is safe?” « DrGDH
I would like to know the date of approval for the use of tPA in Irish hospitals
Hi Rose. I’m afraid I don’t know the specific date for Ireland but it was approved by the European Medicines Agency in 2002 and it was likely similar in Ireland. Sorry I can’t give you anything more specific