This is the transcript, slides and references for a talk I gave at the 2025 Irish Association for Emergency Medicine conference. My own institution oragnised hosted this year and I have to commend the EM team there for the stellar show they put on with record registrations. I contributed nothing to it beyond my presence it must be said!

—
Firstly, thanks for the invite, it’s always an honour to be asked to speak at IAEM and it’s great to see how the conference has developed with the growth of the specialty in the country. For those who don’t know me, I trained primarily in emergency medicine for about 18 years or so before I got a consultant job in the Mater. I now work the vast majority of my time in ICU and maybe do a shift a month in ED. I still feel incredibly proud to call myself an emergency physician because I think it’s EM training that makes me a good intensivist.
I want to spend 20 mins on a favourite topic, that of PE, and now that I’m an ivory tower ICU doctor I can skip all the much more difficult questions of who to work up and who not to work up and instead focus on the crashing, dying patient with PE.
I need to spend a bit of time on teasing out the type of patient we’re interested in. This will be a small sub set of the PE population. The ESC guidelines that we all use will help you a little with risk stratification but I do not think on their own that they’re good enough.
Especially within the intermediate-high risk patients there is a huge spectrum. A little right heart strain on a CT and +ve troponin gets you into the intermediate-high category but many of these patients are on room air texting on their phone while others are circling the drain. Hypotension gets you in the high risk category but it’s a very blunt tool for risk stratifying
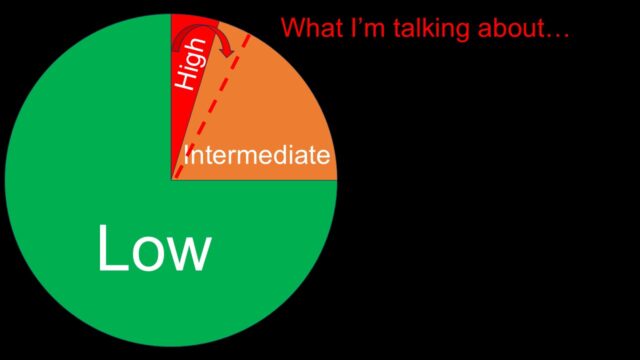
This is a not especially accurate chart of the different types of PE. Most are low risk, a decent bunch are intermediate and a small cohort are high risk.
I think if I was to depict the population I’m talking about they vast majority will be in the high risk or massive group but there may well be a small number hidden in the intermediate or sub massive group.
So see this as a talk that is focused on that very small slice of PE patients that you will see several times in a career and they are defined not so much by the guidelines but by the end of bed o gram and the physiology you can tease out at the bedside. These are the ones that we need to be a little more aggressive in our management

If we’re going to risk stratify further than the ESC guidelines then this is what should we be looking at. This list is a suggestion of where to focus your efforts and we’ll go through each in turn. I’m going to skip the last too in the interests of time but the TLDR is that I think ECG is more helpful as an indicator to do the CT or the echo and the only lab I think is relevant to this discussion is possibly lactate
Let’s start with hypotension. Straight from the guidelines, this is not subtle and available to all of us. If they are actually hypotensive because of the PE then they’re in trouble. The difficulty i find is the blinkers sometimes go on once you have a CT result that says “PE” and a patient with a low blood pressure. The tricky bit is that multimorbid people often get PEs and they often have multiple reasons to have a low BP. For example Is this nursing home patient on their 6th admission this year with urinary sepsis and a blocked nephrostomy now hypotensive because of this segmental PE you’ve found or is it just the urine again.
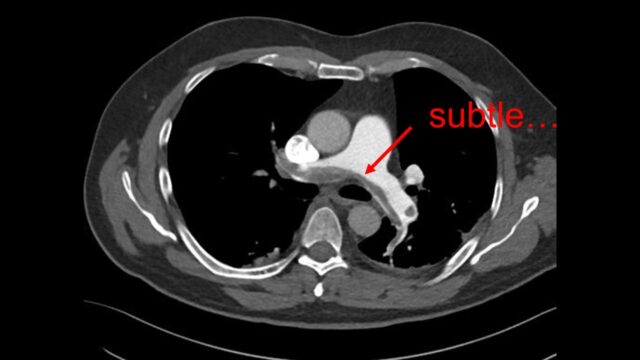
Case courtesy of Frank Gaillard, Radiopaedia.org. From the case rID: 29928
What about the CT scan itself – can this help you tease out the badness?
The classic report is the saddle PE, the huge thrombus straddling the bifurcation of the PAs. Surely this means badness? Clot burden does not make it into the PESI score or into the ESC guidelines. There are a number of papers to support that clot burden does not really predict physiology or outcomes
However I do think that an absence of clot burden suggests that PE is unlikely to be killing your patient. If I see a report of segmental or sub segmental PE in a sick patient then I’m often looking for other pathology to explain why the patient is sick.
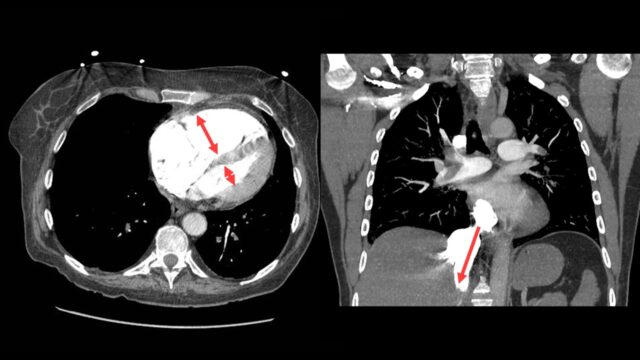
There are two things that I do look for on the CT scan to predict severity the size of the RV compared to the left with the position of IVS
and i look for contrast reflux into the IVC. An RV under acute pressure will fail. When the RV fails it blows out and it fails to pump effectively with > contrast backing up in the IVC. If your radiologists are not reporting this routinely they should be.
The other thing I look for on CT is chronicity. There are lots of patients out there with dilated poorly functioning right ventricles from other causes, mostly chronic lung disease. Some of these patients will also have PEs. Can you distinguish chronic from acute changes?
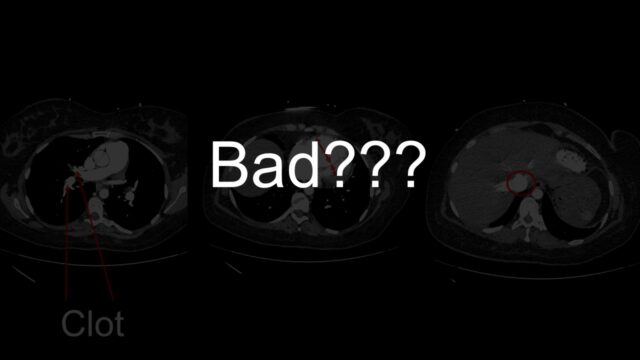
Lets use an example. This a 50M transferred to you from outside hospital with intermediate-high risk PE. You can see the PE here. It’s segmental, it is definitely not big. The RV is massive there is contrast reflux. These are all signs that I’ve been telling you suggest that bad things are happening.
However…
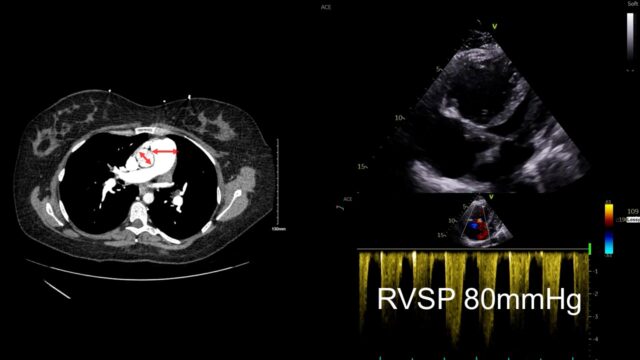
Look further and you can see the main PA is much bigger than the aorta, that takes time to develop. When you look with echo you can see a massive RV but it’s big and chunky and looks like its been lifting kettle bells for years. The bottom is a little doppler way of measuring right sided heart pressures and it suggested that the right heart pressures were far too high for this to be an acute event. This case is actually someone with primary pulmonary hypertension that just happened to have a PE.
I have dedicated more of my career than I would like to admit to echocardiography so it’s hardly surprising that i think you should reach for an echo in PE. My impression from both EM and critical care perspective is that our trainees are now coming out of training with a reliable set of skills to do this. The echo will answer your question – is this hypotensive patient with a PE on CT scan actually dying from PE or is it from something else. I think it’s probably better than CT at teasing out how bad the heart is. It also allows you to assess your therapies to assess if they’re getting better or not.
These images are all from a young female with high risk PE with pre and post lysis images. In a revolution of AI assited imaging they now come with added labels…
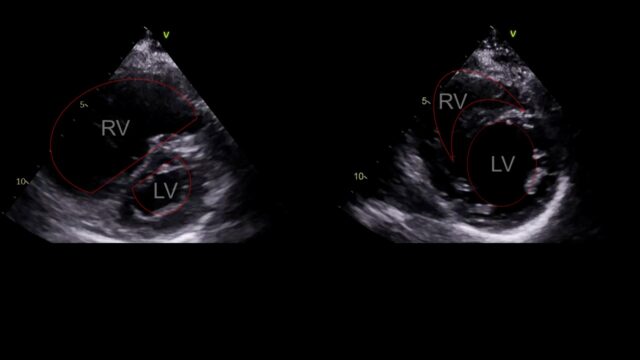
This is PSSAX view. In a life threatening PE the RV enlarges, the right sided pressures rise and the interventricular septum loses its roundness and becomes flat. This is the D sign you might here of. Like a capital D tilted over slightly.
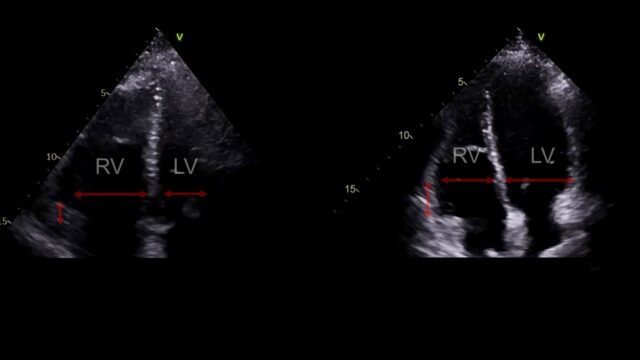
The apical 4 chamber view. The RV should be smaller than the LV, if it’s a lot bigger something is wrong. This view also allows us to look at something called TAPSE basically how much does this little nubbin at the tricuspid valve move up and down. If it moves up and down loads that’s good, if only a little that’s bad.
You can measure lots of other things and as an echo geek I like to do so but in all honesty in this scenario I am not convinced they add anything over what I’ve just shown you
So having spent ages trying to tease out the features and tests that tell us who is super sick with PE can I paint you a picture?

This is direct from our resus room in the mater last month. You can see team work at its very best here. This is caravagio’s “raising of Lazarus” from the dead and I think it communicates what we’re trying to do here
Let’s give more details, they are 65, they look a little clapped out, this might be a colour thing, a work of breathing thing, they might be cold like a cardiogenic shock patient
I’ll even give you some tests, you’ve got them through a CT scan and there is a PE which for the sake of simplicity we will say is one of the main PAs. The RV looks big on the CT scan and there is some contrast reflux. Your echo shows a dilated RV with a very flattened septum and a TAPSE of 0.9cm. The lactate is 4.5. The HR is 135, the sats are 89% on the NRB and the BP is 91/45. The troponin and BNP that you didn’t ask for are both +ve.
The BP I gave you was 91/45 so If we do the ESC risk stratification then they are intermediate-high.
The PEITHO trial from 10 years ago enrolled intermediate-high risk patients. Randomised lysis or not and they had a mortality of 2% in each group.
In real life I think we would all agree this patient likely has a mortality of something much greater than this. I highlight this just to emphasise the bluntness of hypotension as a tool to risk stratify
Let’s make this easy then and let’s say the> BP is now 80/40.
We’re now into the high risk category. >The ESC guidelines suggest heparin, lytics and maybe a surgical embolectomy as class 1 recommendations, all 6 here have a level C evidence base.
Based on the data this is not a terrible list of reperfusion strategies but I think there’s a lot more we should be thinking about. I want to run through a list of things that are likely and unlikely to be helpful things to do in the resus room when your PE patient is dying.

This is a likely controversial list that will come across really poorly without context on twitter or whatever it is people are using these days.
I do really think all of these interventions have a role at certain stages but in the first 30 mins or so of your care of this super sick patient I think their role may either be inappropriate or a distraction.
If blocked pulmonary vasculature is killing the patient then unblocking it is kind of important.
When you look into it there is not high level robust evidence for giving lytics to hypotensive PE patients, most of the trials included a mix of intermediate and high risk patients but this retains a high level recommendation and it is worth dedicating your limited cognitive bandwidth to
If you have a hypotensive PE patient and you are confident the hypotension is caused by the PE then you need to have a very good reason not to be pulling the trigger. I think it is possible to overthink this, possibly because people worry about the very real bleeding issues and perseverate over whether they should pursue clot retrieval or contact a PERT team or something else. All of this will create delay. I’m not saying this is an easy or low risk decision but it is what we are trained for
A variety of regimes exist but I suspect we all use alteplase, commonly 10mg bolus with an infusion of 90mg over a couple of hours.
If someone with PE arrests then giving it during arrest seems prudent. A 50mg bolus is the typical intervention.
You have a lot of options here in terms of anticoagulation. Both LMWH and UFH are valid. Opinions vary on this I might add. However, my preference is almost always UFH. It gives you a lot more options 4 hrs later when they inevitably stop bleeding. As soon as i stop the infusion i know heparin effect will be gone in a couple of hours or less. Once the 1.5mg/kg of LMWH is given you cant take it back and you can’t even really reverse it to any great extent.
How do we support the circulation?
Our default reaction, understandably is to reach for fluids. It is almost always a reasonable thing to do but I’m going to suggest we don’t do that here. If someone is hypotensive in the context of PE then their RV is failing. It is blown out and flaggy. The septum will be squeezed over and impairing LV filling. Dumping a litre of crystalloid into the venous circulation is going to do nothing but make this worse, raising the CVP and pushing the septum further over. This is why I have put fluids in the “not helpful” category
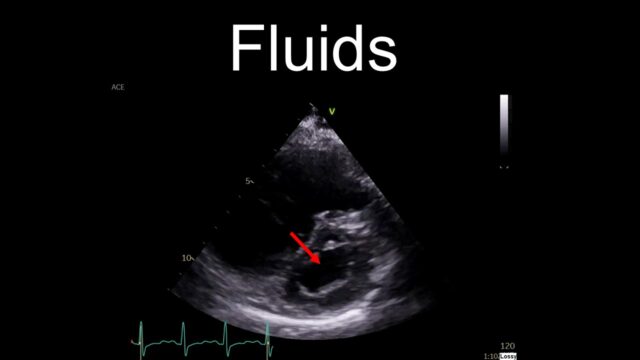
A much better resuscitation fluid to use is noradrenaline. You’ll tighten up the circulation and you get a reasonable amount of inotropy from the norad itself. This is my first go to, I even use it to allow me to get the CT scan on the patient I’m 90% sure has a PE (eg on history and echo etc…) but can’t quite prove yet. It works remarkably well and will often convert an “unstable” PE into something that buys you more time to make a better decision about lysis or an alternative
The sicker they look the more likely I am to reach for God’s own inotrope – adrenaline. If i have echo evidence of a struggling RV in a PE patient and they’re deteriorating in front of me I’ll use some adrenaline in addition to the norad
Of course there are lots of other vasoactives available, they all have their flavours, their pros and cons and they all have their place. But I am trying to keep us focussed on the crashing PE patient in the resus room. Both dobutamine and milrinone despite their theoretical advantages on PA pressures etc cause too much hypotension which you simply can’t afford in this scenario. Vasopressin is another common one touted but it’s not very titratable and it’s more something i’m gonna be reaching for at the 4 hr mark not the first 30-60 mins
I think these drugs are largely a distraction in the scenario we are talking about and fall into the category of nice ideas physiologically but do not fare well in the heat of battle in the first hour in resus
Don’t underestimate this. This is pretty much a free improvement in pulmonary vascular resistance. Hypoxia is a potent vasoconstrictor in the pulmonary circulation which is the last thing you want. Be liberal with the O2. Given the ubiquitous presence of high flow everywhere now this is fairly easy to do.
That being said intubating in the hope of improving oxygenation will not end well. See below…
The cohort I am picturing are sick looking and drowsy and hypoxic. If this was pneumonia then intubation would be an important intervention. For those of us who work in critical care, especially the anaesthetists intubation is like a comfort blanket to us. It’s the one thing we know how to do well and like a hammer looking for a nail we often find an inevitable momentum towards intubation that we find hard to resist.
But intubation here is far more likely to precipitate cardiac arrest and do nothing for your oxygenation. This is another one of those “resuscitate before you intubate” scenarios. Reperfuse before you intubate might be more appropriate.
The cohort i’m describing are all supersick and clapped out and cold. A peripheral is fine to get them through CT but once you have your diagnosis you should dedicate someone to getting lines. Yes there is genuine risk in putting CVCs in the thrombolysed patient but the risk is almost entirely to do with skill. When I watch people do CVCs, I am frequently horrified by the poor needle tracking on display. And this seems to be equally prevalent in the EM and ICM trainees that I work with. Get good at this, i mean really good at this. You are probably not as good as you think you are. When you need it in a hurry in a high risk patient it really matters that you can do it flawlessly and safely.
I’m not trying to be Mr Obvious by saying that CPR is useful if the heart has stopped. I mean particularly in an arrest caused by PE I suspect the CPR has a mechanical effective in breaking up mobilising clot stuck in the RV or the PA and moving it distally hence restoring flow. The typical pattern is the stuttering ROSC with multiple episodes of ROSC as the clot ping pongs around the circulation.
I’d be a little cautious around mechanical CPR devices with alteplase floating around given the data relating to injuries from mechanical CPR
Nitric is probably one of those things EPs are distantly aware but have never used. We use it loads in ICU in the post cardiac surgery and the post transplant population. It is really good at reducing pulmonary vascular resistance and that’s one of the big problems in PE.
There is actually a small RCT of this done by Jeff Kline called the iNOPE trial which used nitric in spontaneously breathing patients via a special face mask. The outcome is in the title iNOPE. Nope it did not work.
if somehow your patient is ventilated then sure, but i certainly wouldn’t add a tube just to do it.
I have put these in the “distraction” because they can be a bit of a distraction either trying to use them on patients who don’t need them or distracting you from the from something more important you could be doing.
The two options realistically are catheter directed lysis where a small catheter is threaded into the clot then they’re given a mg/hr of tPA over 24 hrs. The time scale itself gives you an idea of why it might not be the rescue you think it is for the patient we’re describing. Even in principle I find it hard to see how this better than just siting an IV cannula and giving a mg/hr of tpa. It all ends up in the pulmonary circulation either way. Data here is not wonderful. My prediction is this will fade away over time. HI-PEITHO (which we have recruited for is yet to complete or be published)
The other device is clot retrieval or thrombectomy with the current device de jour being the flowtriever. This involves effectively floating a 25Fr cannula into the clot then using it like a hoover. It is somewhat similar to a VV ECMO circuit but with a filter for the clot and no oxygenator. This is the one that produces all the heavily industry supported twitter images like you see on the right. They always seem like a slightly macabre arts and craft you might do with a toddler.
Again the data is not there yet. The most recent studies i’ve seen are PEERLESS and FLAME. I’ve put some details and discussion on the website if you want to read more. Both leave me underwhelmed and are obviously heavily sponsored.
There is a large trial coming next year that is likely to give us a more definitive answer which I suspect will be – it makes the numbers and the right heart much better but overall outcomes are similar. Time will tell.
I have given this a hard time and you can hear the skepticsm dripping off me here. The theory here is brilliant and we’ve managed to do this for coronary vessels and cerebral vessels so it stands to reason that we should be able to get this right for pulmonary vessels, I just don’t think we’re there yet with the right device and right data and I worry that people will delay giving tPA cause they heard some lad in a hospital down the road might be doing this and they spend 30 minutes trying to find out. Hence why I’ve put these in the distraction category
This is in my distraction category. Which pains me greatly because any day I get to cannulate someone for ECMO is a great day for me. Less so for the patient.
In theory this is great. Effectively you use plumbing to build a bypass completely around the obstruction. You’re taking lots of blood from the right side of the heart and returning it to the aorta without it having to pass through the lungs at all. It is an excellent physiological support for massive PE. You still need to clear the clot somehow so it’s not a stand alone treatment but it really will support the physiology tremendously well
The reason i have it in the category of distraction is that it is almost certainly not available. We are the only centre in the country that does it and we have done it but the timing and logistics of it mean we can only realistically deliver it in goldilocks conditions of time of day and staff availability etc. Jo has spoken already to the complexities of it
It gets talked about in papers and guidelines but you’re talking about taking someone who is already mostly dead into theatre, lined, anaesthetised, chest opened and onto bypass. There probably is a role for it somewhere and in certain institutions but it’s often raised in the context of contraindications to lysis but those same contraindications to lysis usually apply to the 30000 units of heparin you need to get them on bypass.
In terms of data there are a number of case series published with variable outcomes which seem to correlate with patient selection with many patients seeming to me like they could have been managed just fine without the operation IMHO. It also ties you into a long tedious discussion and waiting period while a surgeon decides whether to do it or not and you’re always damned by the goldilocks issues of “not sick enough” and ”too sick for the operation”
Finally I put PERT teams in the distraction category. And I feel bad about that because they’re usually filled with knowledgeable and enthusiastic people . But there are 2 errors I’ve seen on this.
One is on us where we outsource the decision to lyse in someone who has a clear indication. This is not necessarily the fault of the PERT team but there is risk to the patient in delaying as it is a tremendous faff trying to get hold of the relevant people and then get them to agree
The second distraction that can happen is the recommendation for interventions in a patient that they have not seen and are not present to. A couple of times I have had to stand down IR interventions that frankly were not needed because the patient was getting better with conventional treatment. Perhaps I don’t have to tell a group of emergency physicians this but – Do not underestimate the importance of being at the bedside and seeing the patient.
Below are a few extra slides and discussion I had to cut due to time constraints. Added here for completeness
The ECG in risk stratification
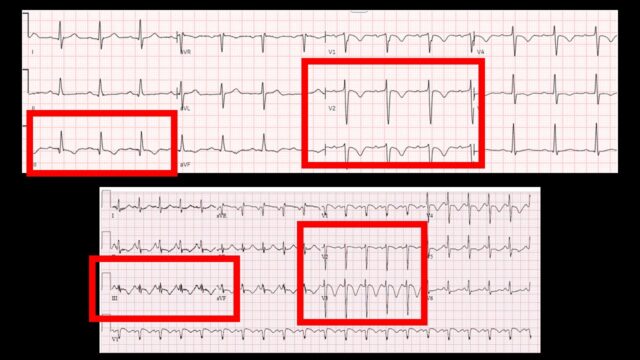
I find myself not looking at the ECG that much in this scenario. we often look at the ECG with a STEMI no STEMI perspective and then set it aside while we wait for the CT scan but there are some nice and moderately specific signs on ECG that suggest that there is right heart strain. Here are 2 examples from steve smith’s ecg blog. Key findings being the TWI in V1-3 but also lead III. Note the small r wave and big s wave. The ECG (like the echo) won’t give you the cause of the right heart strain but it is the electrographic correlate of what we were just looking at on echo and CT.
It’s advantage is its very easy to get and will probably already be done, the disadvantage is I think we all struggle a little to interpret it
ECGs above are from Steve Smith’s Blog of course!! Cannot recommend highly enough
Labs in risk stratification
The guidelines have both trop and BNP in their risk stratification algorithm but the trop is so sensitive that the it turns +ve in lots of well looking patients so I just don’t find it discriminates well in the short term.
If you want to look at a blood test to predict badness then i’d probably opt for our universal badness meter in the form of the lactate level.
How long should you continue CPR once lysis is given
This is a little of a personal hobby horse.
If someone with PE arrests then giving alteplase seems prudent. A 50mg bolus is the typical intervention. The ACLS recs sugges consider CPR for 60-90 mins. This is frequently misinterpreted that you must do CPR for 60-90 minutes I think this is a little mad and when you chase down the references are really not very supportive.
The 3 studies cited by resus guidelines for the consider 60-90 mins CPR recommendation
- lit review pulling out cases to a total of 30.
- Most PE were not proven (this was mid 90s)
- urokinase or streptokinase was the agent
- there’s a statement that up to 90 mins CPR was done but also that most lysis was given quite delayed before it was initiated.
- here are no specific details
- there was a fair amount of bleeding
- Case report. 70M lysed empirically during CPR. CT confirmed PE after the fact.
- had a stroke and some hemorrhagic transformation in the week after and did OK.
- single centre chart review with limited methods
- 22 patients, mostly in patients
- only 3 had confirmed PE prior to lysis, all had ROSC
- only 3/22 survived and it took 30 mins to give the tpa
On the other hand a real life data set said ROSC, if it’s going to happen generally happens before the 15min mark . Whether or not the ROSC was linked to the tPA is hard to prove
Ultimately I think how long you do CPR should be based on other things rather than this empiric 60-90 minutes which does not seem supported in my view.
Data on catheter based devices
This is neither systematic or comprehensive so take it for what it is.
- Methods
- non randomised data on high risk PEs (important in itself)
- no protocols here directing treatment, could get whatever physician thought best
- primary outcome was a composite which is less than ideal
- they compare flowtriever vs other interventions
- unded by the manufacturer
- Results
- 115 pts in 11 sites over 18 months.
- more flowtriever patients had some kind of contraindication to lysis
- more severely shocked patients in the control arm
- in control arm 2/3 got lysis, a quarter just got anticoag alone and a small number got CDT
- median time to reperfusion strategy was 6 hrs in flow triever arm and 4 hrs in control arm which seems long for both!
- 2% vs 30% mortality favouring flow triever but this is of course horribly confounded.
- major bleeding in 10% vs 25% favouring flowtriever (but it still seems quite high)
- Thoughts
- nice to have some observational data but the lack of randomisation means it’s hard to know if its meaningful
- Methods
- title says RCT of mechanical thrombectomy vs CDT in intermediate risk PE. However the inclusion criteria suggest this is more like intermediate high (which seems very appropriate)
- thrombectomy was the big flowtriever device. CDL was the EKOS
- Primary outcome was an impenetrable composite ratio
- Sponsored by the device manufacturer
- Results
- 550 pts
- about 24hrs from presentation to device placement which gives you a sense that these are semi-elective procedures after everyone has had a good think.
- major bleeding similar in both
- mechanical much better on their “win ratio”
- mechanical longer to do (90mins) but no indwelling catheter left. CDT quicker (60 mins) but catheter stays for a day
- mortality was too low to judge a difference but it was effectively the same 0.4% v 0.8%
- they measure an awful lot of things to see if there was an improvement but they aren’t the outcome we’re looking for.
- ICU stay is part of their outcome but that seems like cheating as by definition the CDT needs a 24 hr stay. It’s not the physiology so of course it will look worse.
- Thoughts
- needed a control arm given that PEITHO had already suggested that even lytics don’t seem to help the intermediate group.
- underwhelming trial
- Methods
- this is the EKOS (US assisted CDT)
- systematic review
- includes both case control and RCT which are hardly the same
- Results
- 28 studies
- 2100 pts
- haemodynamics get beter
- 3% hospital mortality in those who got CDT
- Thoughts
- mostly not randomised which massively limits conclusions
References (in no particular structure)

Pingback: LITFL Update 054 • LITFL • Newsletter