 This is part 2 of a series of posts considering the evidence for thrombolytics in stroke.
This is part 2 of a series of posts considering the evidence for thrombolytics in stroke.
Part one can be found here
SITS-ISTR and SITS-MOST
You may have seen these two trials. The most important thing to be said about these is that they are not trials.
They are reports of voluntary registry data (caveat – the european license dictated the setting up of these registries but that doesn’t mean it’s enforced) of patients who recieved thrombolysis. This is purely observational data of patients who someone thought should get tPA.
Despite this the authors do multiple comparisons with historical controls – chosen favorably from amongst the prior trials.
This is comparing apples and oranges. I hope it’s clear that the patients getting tPA in the SITS papers are different patients than those in the other papers. Therefore it is not fair to claim comparable efficacy.
This is a major objection to the thrombolytic literature in general, and in particular it’s combination in meta-analyses. The NNT summary covers this idea well.
The ATLANTIS trials
The atlantis trial was presented in two parts. Initially it was intended to be one trial, looking at tPA form 0-6 hours.
Two things happened:
- Once the NINDS study was published, the investigators scapped the idea of giving placebo to the o-3 hr patients as this was considered unethical.
- the safety monitoring committee found concerning results in the 5-6 hr patients given tPA.
Thus Atlantis A was a trial of 0-6 hrs that was stopped and Atlantis B was a re-launch taking patients at 3-5hours.
ATLANTIS A
Patients
had similar inclusion/exclusion criteria to NINDS, but allowed for the investigators to exclude patients with “Any other condition that the investigator feels would pose a significant hazard to the patient if rtPA therapy were initiated”
Outcomes
They had two primary outcomes (again this is a little bit naughty)
- a reduction in NIHSS at 24hrs and 30 days
- a reduction in infarct size
I would contend that both of these outcomes scream SURROGATE MARKER.
Results
most were thrombolysed after 4 hrs. This is understandable – it was the early 90s after all; there weren’t many stroke teams about.
The strokes were less severe (a trend in every trial since NINDS) with a median NIHSS of 10 or 11 (it was 14 in NINDS)
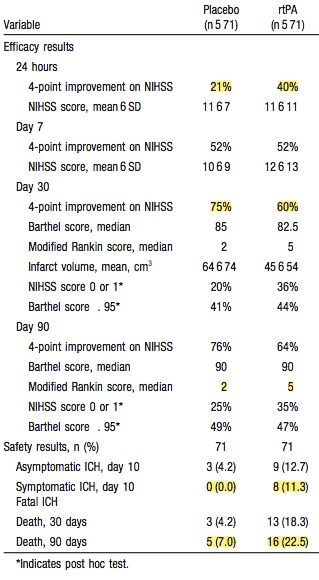
Of the patients with an NIHSS>20 who got tPA every one of them (16 of them) was dead by 90 days.
I hope you’re convinced that this was a thoroughly negative trial by now
ATLANTIS B
Patients
They took patients from 3-5 hours and changed the inclusion criteria subtly. Patients with a CT showing greater than 1/3 of the MCA territory involved were excluded. The basic effect is to exclude the bigger strokes. This is now ubiquitous in the newer trials.
Outcomes
they had the ambitious primary outcome of an NIHSS of 0-1 (on a 42 point scale!) at 90 days. In the secondary outcomes they had the modified rankin scale that we’re probably more interested in.
Results
they recruited 600 patients, most of whom who got lysis at about 4 hrs or so.
Again they were less severe strokes – NIHSS of 10 in this trial.
Their primary outcome makes fascinatiing reading
- 32% of placebo patients and 34% of tPA patients had an NIHSS of 1 or less at 90 days.
That deserves comment, even though it’s a recurrent finding in all the trials. People with strokes sometimes do great no matter what we do. Or perhaps more accurately. People with strokes will often (if you count a 1/3 of the time as often) do really well with good nursing care and good stroke management. Let me quote the cochrane review of stroke units (comprising, dedicated nursing, medical, OT and physio and feedings care.)
Stroke patients who receive organised inpatient care in a stroke unit are more likely to be alive, independent, and living at home one year after the stroke
Over all the studies the placebo group often do well (MRS <2 in 35-45% of placebo patients). We all know people with severe strokes who are “prisoners in their own bodies” but this is probably not as high a percentage as you thought it was. They are also not likely to be eligible for tPA anyhow (NIHSS greater than 25 or a nasty CT)
As for secondary outcomes, like that pesky one – death, placebo won with mortality of 7% for placebo and 11% for tPA
These trials are important when it comes to reading ECASS III as it claimes postive effects on patients treated beyond the 3 hr mark, whereas these trials showed that people did really poorly beyond the 3 hr mark.
Comments as ever are welcome.
References and further reading.
If you fancy looking at some CTs with subtle early stroke signs then these sites have some useful training:
The RCTs covered in this post:
- Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999 Dec.;282(21):2019–2026. PMID: 10591384
- The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g) : results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke 2000 Apr.;31(4):811–816. PMID 10753980
The other RCTs
- Randomised controlled trial of streptokinase, aspirin, and combination of both in treatment of acute ischaemic stroke. Multicentre Acute Stroke Trial–Italy (MAST-I) Group. The Lancet 1995 Dec.;346(8989):1509 -1514. PMID: 7491044
- Thrombolytic therapy with streptokinase in acute ischemic stroke. The Multicenter Acute Stroke Trial–Europe Study Group. N Engl J Med 1996 Jul.;335(3):145–150. PMID: 8657211
- Streptokinase for acute ischemic stroke with relationship to time of administration: Australian Streptokinase (ASK) Trial Study Group. JAMA 1996 Sep.;276(12):961–966. PMID: 8805730
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995 Dec.;333(24):1581–1587. PMID: 7477192
- Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995 Oct.;274(13):1017–1025.1. PMID: 7563451
- Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. The Lancet 1998 Oct.;352(9136):1245–1251. PMID: 9788453
- Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008 Sep.;359(13):1317–1329. PMID: 18815396
- Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurology 2009 Feb.;8(2):141–150. PMCID 2730486
- Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurology 2008 Apr.;7(4):299–309.PMID 18296121
Pingback: Evidence for thrombolytics in stroke – Part 3 | Emergency Medicine Ireland
Pingback: The LITFL Review 024 - Life in the FastLane Medical Blog
Pingback: Evidence for thrombolytics in stroke – part 4 | Emergency Medicine Ireland
Pingback: Evidence for thrombolytics in stroke – part 5 | Emergency Medicine Ireland