I have been speaking at EuSEM for about 5 years now and it’s lovely to finally be back in person.
This is a summary of the talk I gave at the EuSEM 2021 conference in Lisbon. I’ll add a screencast in due course.
Case 1 – Drug induced neutropaenia
Mrs Young is a 35 year old female who is new to the area and has been brought into the ED because she has been behaving strangely and there was concern for a mental health crisis. There is no one available to give a collateral history. When you meet with her she is pacing the room and complaining about the nanobots that her enemies have implanted in her mouth. She has tin foil wrapped around her head to try and stop the same people stealing her thoughts. You eventually obtain some vital signs and find the following
T 38.1, HR 105, BP 110/55, RR 18, Sats 92%
She has been seen by your psych team also who feel she needs involuntary admission for her mental health but want to be sure she is medically fit to have a psych admission. The department is heaving and you’re keen to get her to a destination but is it safe?
Case 2 – Oesophageal ca with neutropaenia and home
Mr Smith is a 72 year old male who is undergoing neoadjuvant chemotherapy prior to a hopefully curative resection of a recently diagnosed oesophageal malignancy. He is 10 days after his last cycle of chemo and this one has hit him pretty hard. He had managed some light gardening during the previous cycles but this one has left him floored. He spiked a fever tonight and came to the ED. His temp was 39.2 on arrival but is now 37.8. He examined unremarkably apart from some mild oral mucositis. A CXR was normal and his vtial signs were unremarkable. His neutrophil count comes back 0.6. You’re preparing to admit him but he tells you he’ll do anything to get home as his son is visiting for a day or two with his new grandchild and doesn’t want to miss out seeing them. Surely we admit all the febrile neutropaenia patients? Don’t we?
Case 3 – Haematological malignancy with neutropaenia and crashing
Mrs Jones is 45 years old with a recently diagnosed diffuse large B cell lymphoma . She is deep in the midst of her chemotherapy and has had two admissions already in the past few months with a stay in the ICU. She mentions something about R-CHOP chemo to which you nod along politely pretending you know what that means. She has a long term vascular access device in place. She presents from home looking terrible. She is pale, nauseated, shut down, tachycardiac and hypotensive. Temp is 39.4. She had some bloods checked yesterday as an OPD and when you look at the neutrophil count you see a big round zero.
We’ll start with some basic definitions. When you get your full blood count back you typically get a white cell count. If you’re lucky your lab will have an automatic typing on that and it’ll give you a breakdown of the different types of white cells. What we’re interested in is the ANC, the absolute neutrophil count. Most sources say that neutropaenia begins at 1500 or 1.5 but in general most of the guidelines consider severe to be <500 and profound to be <100
Fever in these guidelines is typically described as >38.3 but I think given the uncertainty around the accuracy of home thermometers in particular i would take any fever seriously.
What is that neutrophils do and do we really need them? I’d love to say this is a refresher from medical school but honestly I’m pretty sure I didn’t know this in the first place. Neutrophils form a sort of first responder of the immune system, being highly mobile and communicative they arrive at the site of infection. When they arrive they can eat the bacteria, or give of a bunch of cytokines asking for help or even produce something called the neutrophil extracellular trap which sounds super cool. Ultimately a deficiency leads you prone to infection with some pretty nasty bugs with the leaders being with both gram +ve and GNBs in a close race for leading cause of infections in these populations but the fungi have their only special place in the neutropaenic population.
Let’s turn to case 1, our young female who appears to have an exacerbation of an underlying psychiatric disorder. There is one very pertinent piece of information that i did not give you in the vignette is that this patient is on clozapine.
There are of course multiple drugs that can cause neutropaenia, but clozapine is so renowned for it that many countries have specialised clinics allowing regular checking of the WCC to ensure that the patient doesn’t develop this potentially life threatening combination. The mechanism here is unclear but probably involves two mechanisms 1) a drug induced immune activation where the immune system is stimulated by the clozapine to produce antibodies directed against neutrophils 2) there probably is some direct toxic effect on the bone marrow that suppresses neutrophil production. Either way the patient is left extremely vulnerable to infection.
You might pick up a drug induced neutropaenia in a variety of ways, as part of a routine screening programme that is commonly done in clozapine programmes or more significantly when a patient presents acutely. Acute presentations can often include oral ulcerations.
Drug related febrile neutropaenia is less common and less well studied than chemo related versions. There are no good guidelines or RCTs out there to support you but it would seem reasonable to approach management of febrile neutropaenia in these cases in a similar fashion to how you would for any typical febrile neutropaenia case. The real challenge for us in EM is awareness. The list of drugs that can cause neutropaenia is huge. I think the psych patient is the real challenge as they are often not able to communicate what drugs they might be on and they often present out of hours and outside the usual catchment area so you might not have all the previous documentation. Overall they’re a vulnerable group of patients. “Medical clearance” for psych review is something that often causes us a real headache but this is one group where it’s worth taking the time to ensure the patient is safe.
In our case when you looked in the mouth to look for the tracking nanobots you saw multiple oral ulcerations. Between that and the fever, you put a hold on the transfer to the psychiatric facility and instead got some bloods taken. Ultimately the neutrophils came back at 0.2 a CXR showed some patchy inflammatory changes. She ends up being admitted on broad spectrum antibiotics to the medical team with her clozapine held. Before the psych team leaves for the night you’re sure to get some recommendations on alternative anti-psychotic medication to ensure her acute psychosis is well managed in addition to her neutropaenic sepsis.

The commonest presentation we see of febrile neutropaenia is in cancer patients, typically cancer patients who have recently been in receipt of chemotherapy.
Chemotherapy is a whole world of complexity that is changing incredibly rapidly, and frankly as a generalist emergency physician it would be impossible to keep up with the sheer number and type of regimes available. It’s probably worth splitting them up into very broad categories to help guide you.
Hormonal therapy – perhaps the best examples being drugs like tamoxifen for breast cancer. Tamoxifen is a cancer therapy that a patient might describe as chemo but in reality there is no risk of neutropaenia
Targeted therapies – these are the new kids on the block. The molecularly targeted therapies that look like science we’re more used to seeing on star trek. Included here are the immunotherapies and many of the monoclonal antibodies ending in the “mab”. These often involve some form of magical trickery with the immune system getting the immune system to target the cancer. They have all their own complications, many of which are life threatening but neutropaenia is not typically one of them.
Cytotoxic chemotherapy – this is the original gangster of chemotherapy, These agents are used to inhibit cell division in an almost indiscriminate fashion with the hope of wiping out the tumour before you wipe out the patient. The bone marrow and the gut are particularly sensitive to this type of agent. As a result neutropaenia is really common and pretty much an expected complication of therapy. The gut, being the interface between the outside world and the inner workings of the human being plays a key role in keeping bugs out of the blood stream. When cytotoxic chemo damages the gut epithelium then the host is wide open to all kinds of bacterial translocation. The combo of no neutrophils and a leaky epithelium primes the patient for episodes of neutropaenic sepsis. Indeed, while we’re used to looking at the lungs and the urine for sources of community acquired sepsis, in the neutropaenic patient it’s usually bacterial translocation that is the source with little to find on imaging.
After a little bit of basic science then let’s turn back to our cases.
Case 2 you’ll remember was Mr Smith – our relatively well appearing gentleman with esophageal ca who is undergoing chemo as preparation for an oesophagectomy in the coming weeks. He received traditional cytotoxic chemo and has gone through many of the usual side effects of nausea and hair loss but he was warned about fevers and has now presented for evaluation. As noted he looks quite well and wants to go home.
Mr Smith is a great example of someone we want to risk stratify. We do this a lot in EM, we do it with chest pain, we do it with outpatient PE management. Traditionally, in all of the many EDs I have worked, fever and neutropaenia will buy you a straightforward ticket to admission. We would ring an oncology service and they would invariably advise admission even for patients like Mr Smith who looks great and wants to go home. Can we do better than that. Can we safely risk stratify patients like this to a safe discharge? The answer is almost certainly yes. There are of course lots of systems and safety netting and follow up issues to be addressed but the literature supporting risk stratification is reasonable.
Competing for dominance in this space are two scores: MASCC and CISNE. Like all scores none are perfect and they both come with caveats but they are both validated and they are a reasonable tool to add to your clinical judgement in these cases. If we look at the score for both we see Mr Smith comes in low risk on both.
In general the key features that distinguish low risk are.
- Type of malignancy with solid tumours being much lower risk than haematologiacal malignancies,
- Expected duration of neutropaenia – again patients with solid tumours will have a much more rapid bounce back in their neuts than someone who has just had a stem cell transplant.
- Their symptoms – someone who looks well with nil on exam, who was febrile due to a transient bacterial translocation from the gut is much lower risk than someone with a new oxygen requirement with a pulmonary infiltrate.
Ultimately we check both MASCC and CISNE scores on MD Calc and after a discussion with oncology Mr Smith is delighted to go home on some oral co amoxiclav with safety netting an OPD appointment in 2 days with his oncologist
Our last patient of the night shift is Mrs Jones, if you remember we were seeing her in the resus bay and things were not going well. She is clearly critically ill and she’s really in trouble. Mrs Jones ticks all the boxes for a high risk presentation. She has received a fairly potent type of chemo – R-CHOP being a mix of cytotoxic chemotherapy, cyclophosphamide, hydroxydaunorubicin, oncovin (vincristine) with a chaser of prednisolone for good measure. The R in R-CHOP refers to Rituximab – one of the “mab” targeted cancer therapies we mentioned above. Rituximab is a selective antibody that kills CD20 B cells – B cells being a power house of antibody production in the human body. As a result poor Mrs Jones is faced with the burden of not only severe neutropaenia but also a non responsive humoral immune system.
So that’s the background – how are we going to approach this resuscitation?
I think the important take home is to approach it like any other sepsis resuscitation – this is something we do commonly and we do really well. Pay attention to the ABCs and be aggressive in your management.
The presentation here is mainly of heamodynamic collapse secondary to sepsis rather than respiratory failure and while she will likely end up intubated it would be wise to spend some time resuscitating before you intubate.
One of the big issues in these patients is getting appropriate coverage for all the bugs. Given that the commonest source of bacteraemia in these patients is bacterial translocation then gram negative sepsis is your main concern. Think of all the pseudomonas and e coli that could be running around this lady’s bloodstream. Agents like pip-taz, cefipime and even meropenem would all be a reasonable start. There’s clear benefit to addition of an aminoglycoside in the shocked gram negative bacteraemia patient so ensure some gentamicin or amikacin gets given and personally I would give the big dose and not adjust for renal dysfunction as this lady is highly likely to end up on CRRT no matter what you do.
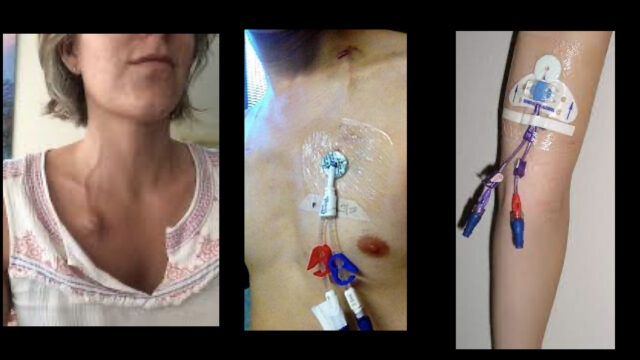
The additional complication we have here is the line. Line infections can grow a mix of bugs including all the same gram negatives from the gut in addition gram positives like staph aureus or staph epis. Frequent empiric recommendations will be addition of something like vancomycin if there is any concern about the line. Chemo patients often have a variety of central venous access devices to facilitate administration. The simple devices might be a run of the mill PICC line but more commonly they’ll have some kind of tunnnelled device. In the ED there is no way you’ll be able to tell if the line is the source unless there’s something like pus coming from the site. People often recommend removing these lines but be wary of that recommendation as it’s often a trap! The tunnelled lines all have these fibrous cuffs that are designed to get enmeshed in the subcutaneous tissues meaning that they need manually dissected out. This is not a task to take on in a haematological patient with no platelets in the ED! However if they had a simple PICC line that’s been in for a while I would very happily pull that out once I have obtained definitive central access somewhere else.
Your hospital will undoubtedly have a protocol for neutropaenic sepsis that will usually include an antipseudomonal penicillin or a 4th gen cephalosporin in addition to an aminoglycoside. This will cover the majority of patients with moderate disease but for someone like this I would phone a microbiologist or ID specialist – whoever it is in your hospital who looks after these complex types of patients.

It’s worth mentioning a brief word on fungal infections. Candidaemia is a particular concern in these types of patients but it is typically more of a problem for the inpatient population who have been neutropaenic for prolonged periods who have already been on antibiotics for several days. I use an awful lot of caspofungin in my ICU job but as yet have never prescribed it in the ED. If you do give a broad spectrum antifungal in the ED then this is probably the patient to use it on.
If you’re still struggling for a clear source or if the patient’s symptoms suggest it then personally I would endeavour to get a CT. While we know that most of sepsis here is from translocation, occasionally you’ll find patients with things like typhlitis or c diff colitis as the source. Conditions which occasionally require surgical intervention. I certainly would not compromise someone’s resuscitation to get a CT but with my ICU hat on this is often one of the first things I’ll be ordering when they arrive in the ICU. This is not a population where you should be worrying about over imaging or radiation exposure. I know it’s not on the image but I would scan the head too. Haem patients typically have no platelets and you often dfind cerebral lymphomas or bleeding so it’s always worth adding on in someone this sick.
Finally a word on G-CSF. This is a magical medication that acts like a drill sergeant screaming at the bone marrow to create more neutrophils. It is sometimes used as prophylaxis for those at high risk of neutropaenia but when it’s been studied in those who have developed febrile neutropaenia the results have been underwhelming.
The take home is simply not to worry about it in the ED as it is not recommended.
You dive into the resus for Mrs Jones, obtaining a separate IJ central line and starting vasopressors and broad spectrum antibiotics. You remove the PICC line and identify a bilateral pneumonia and after stabilizing, proceed to a haemodynamically stable RSI and get her transferred to the ICU passing through the CT scanner on the way there.
So in summary
Not all febrile neutropaenia is in cancer patients – drug induced is a real thing and while much lower risk it still needs careful management
Some febrile neutropaenia patients can be safely managed as out patients. Low risk features include clinical appearance and patients with solid tumours. The MASCC or CISNE score can be helpful in risk stratifying
Haematolical neutropaenic sepsis patients are some of the sickest you will ever see. Apparopraite antimicrobial coverage is key and I would seek expert help if you can get it. Resuscitate aggressively and think about source control.
References:
- UTD articles carried a lot of weight for this talk. As a non specialist in the area I am more than happy to do that!
- REBEL EM
- LITFL
- White, L. & Ybarra, M. Neutropenic Fever. Hematology Oncol Clin North Am 31, 981–993 (2017).
- Zimmer AJ, Freifeld AG. Optimal Management of Neutropenic Fever in Patients With Cancer. J Oncol Pract. 2019 Jan;15(1):19-24. doi: 10.1200/JOP.18.00269. PMID: 30629902.

Brilliant summary. As usual. Thanks