Lytics in stroke
This has been an interest of mine for a while. So much so that I’ve gone and written a slightly meandering epic on it all…
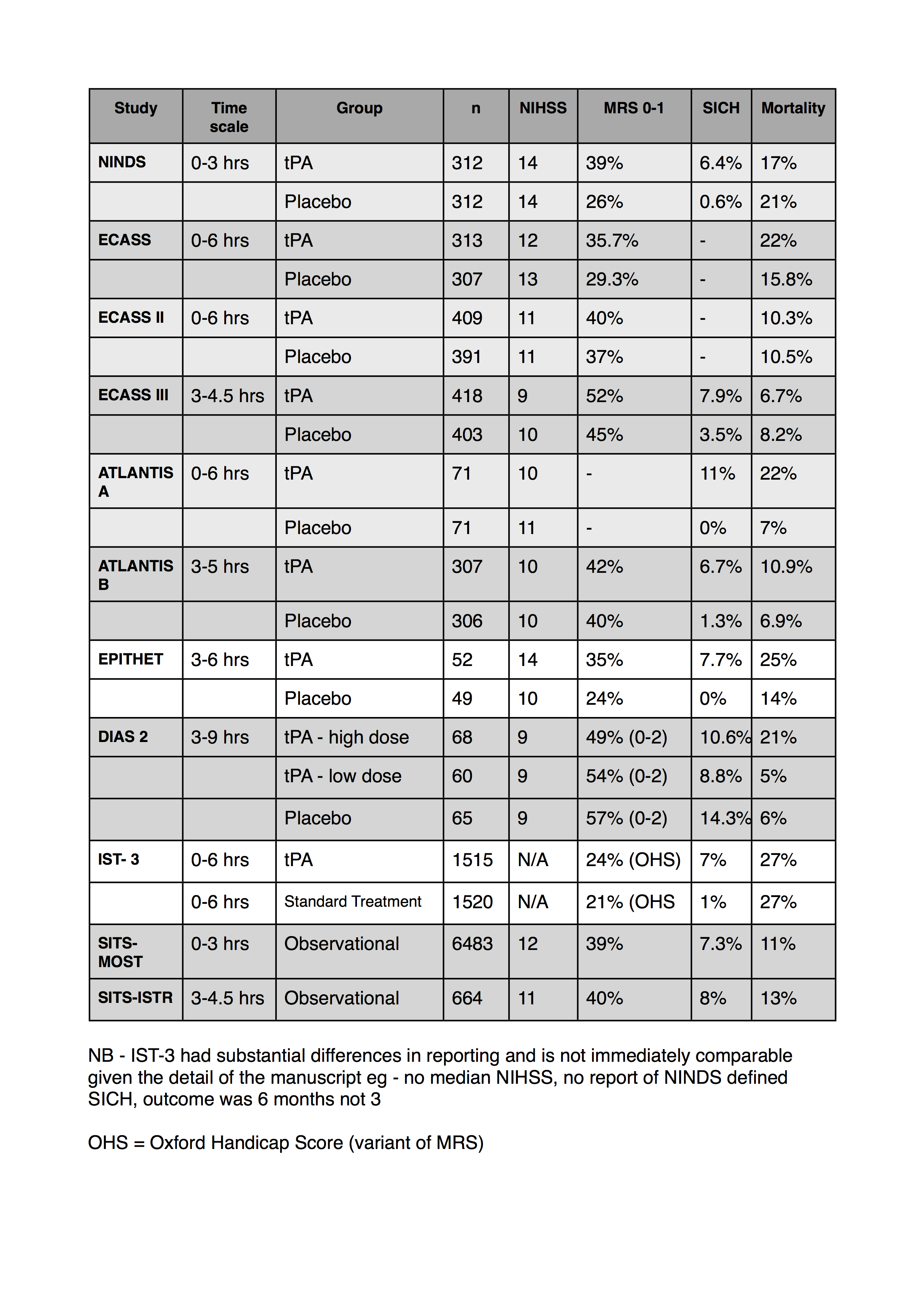
I’ve also collated a table of some of the numbers from the studies regarding outcomes and adverse events. I’ve tried to use the NINDS definition for SICH if I can find it in the corresponding paper. Let me know if I’ve got any of the numbers wrong.
I’ve added my thoughts on IST-3 at the very bottom and updated the table below.
Note:
- I can’t find MRS data for the atlantis A trial
- only the most recent ECASS data reported a NINDS definition of SICH
- the SITS studies are both observational registry data
References:
The RCTs
- Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurology 2009 Feb.;8(2):141–150. PMCID 2730486
- Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurology 2008 Apr.;7(4):299–309.PMID 18296121
- Randomised controlled trial of streptokinase, aspirin, and combination of both in treatment of acute ischaemic stroke. Multicentre Acute Stroke Trial–Italy (MAST-I) Group. The Lancet 1995 Dec.;346(8989):1509 -1514. PMID: 7491044
- Thrombolytic therapy with streptokinase in acute ischemic stroke. The Multicenter Acute Stroke Trial–Europe Study Group (MAST-E). N Engl J Med 1996 Jul.;335(3):145–150. PMID: 8657211
- Streptokinase for acute ischemic stroke with relationship to time of administration: Australian Streptokinase (ASK) Trial Study Group. JAMA 1996 Sep.;276(12):961–966. PMID: 8805730
- Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS (B) Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999 Dec.;282(21):2019–2026. PMID: 10591384
- The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g) : results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators.(ATLANTIS A) Stroke 2000 Apr.;31(4):811–816. PMID 10753980
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (NINDS). N Engl J Med 1995 Dec.;333(24):1581–1587. PMID: 7477192
- Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995 Oct.;274(13):1017–1025.1. PMID: 7563451
- Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. The Lancet 1998 Oct.;352(9136):1245–1251. PMID: 9788453
- Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke (ECASS III). N Engl J Med 2008 Sep.;359(13):1317–1329. PMID: 18815396
- The IST-3 collaborative group. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012 May 23.PMID: 22632908