OK so IST-3 is out. It’s a big and important trial so make sure and read it. Stroke lytics get a lot of attention from EM folks, if only because we seem the only ones not convinced of its efficacy. Jerry Hoffman is probably the most important contentious voice but he’s by no means alone. Ryan Radecki has also some good stuff online and in print about it. I’ve even got my own compendium on it.
When I heard IST-3 was underway and almost ready to report I was quite excited to see someone finally answering the question in a rigorous way – do lytics in acute stroke improve outcomes? The trial that is still cited is the original NINDS trial. It showed a benefit but it was small (comparatively) and there were baseline differences between the groups that may have biased the trial in favour of tPA. This could have been answered by repeating the trial and replicating the results (a fairly common practice). This has never been done and unfortunately IST-3 is not the one to do it either. Now that’s hardly surprising – it was never the question the authors set out to answer.
If you don’t fancy reading the more detailed analysis below here’s the three major problems my point of view.
- this is an open label trial – It was blinded at assessment but in hospital following treatment everyone knew who got tPA and who didn’t and they were treated differently. This is a potential source of bias
- it only randomised people currently outside the licence for tPA so it’s testing an entirely different bunch from NINDS. See here for which patients were randomised.
- it was a negative trial by its primary outcome. Something the authors don’t acknowledge in the conclusion in the abstract.
There are lots of other problems that I’ve highlighted below. I’d be interested in your thoughts.
METHODS
- multicentre international RCT
- a pilot phase (<10% total) was blinded and then in the main trial it was open label. They don’t explain why the main trial was open label but presumably it’s cheaper and easier to do.
- Randomised on basis of uncertainty principle. Basically if you’re unsure whether or not you should give it based on current guidelines then randomise
- all kinds of follow-up but mainly via GP or phone or mailed questionnaire
- originally planned for 6000 but recruitment insufficient so recalculated their power and changed the statistical plan.
- realised that there were big differences in the sub-groups at baseline (mainly on time and stroke severity) and had to apply logistic regression to adjust for them. Seeing as the only +ve results in the trial were in the sub-groups it makes me further question their significance if they were adjusted to compensate for base-line imbalances.
RESULTS
- 3000 over 10 years (which is very slow) 300/yr split between 150 centres means 2/yr/centre
- half over 80 years old
- virtually all outside the current european licence (which is the point of the trial)
- mainly treated at 4.2hrs
- pts who got tPA more likely to go to HDU (24%v17%) than those who didn’t. For this you could perhaps make the assumption that pts who got tPA got better nursing care…
- big spike in early deaths (11% v 7%) but then improved by 6 months (16% v 20%). Overall mortality was identical at 6 months
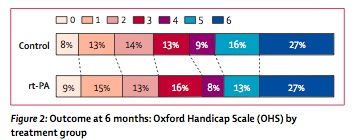
- found a 2% benefit in primary outcome (alive and independent, 35% v 37%) at 6 months. A difference this small of course did not reach statistical significance. You could call this an NNT of 50 if it was real.
- significant ICH 7% v 1%
- oddly an increase in fatal swelling (odd because if tPA works then the infarct would be smaller and the swelling would be less) of infarcts in tPA group of 47 pts v 25 pts. This is played down as inconsistent with prior studies in the paper. UPDATE: it was pointed out to me by a fine neurologist that when you lyse the clot you get reperfusion oedema so this is actually a sign the tPA does breakdown clot. It makes sense. It’s still a problem if the reperfusion oedema kills people, but it’s not oedema simply from a big infarct.
- they have a whole ream of things in the forest plot of secondary outcomes (these are the adjusted ones) and only one approaches significance – age >80 yrs. Unfortunately as Ryan points out – if it’s better for those greater than 80 then it’s worse for those <80 – which is the precise opposite of prior trials.
Below is a video of the lead author talking about the results if you’re interested
http://www.youtube.com/watch?v=E9oRXu2ORCY&feature=g-hist
The paper itself lives here
In the same issue the same authors have published an updated systematic review and meta-analysis that now includes these results. One of the concerns that has been pointed out before is perhaps this heterogeneous data set (now up to 12 very differently ran trials) aren’t actually appropriate to meta-analyse.
There’s a glowing editorial about the trial that makes the slightly odd and even reckless statement that:
Every stroke patient should therefore be classed as a candidate for thrombolysis
This seems a little bit of a stretch seeing as the IST-3 trial used these criteria and excluded lots of pts:
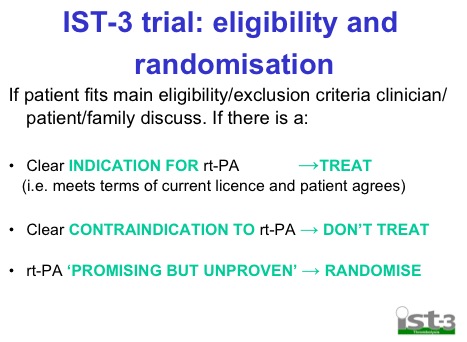
My suspicion as always, is that tPA does work for some patients with stroke, but certainly not all and until we can pick out the ones who benefit then I suspect that we shouldn’t have adopted this as wholeheartedly as we already have.
UPDATE – if you look at page 4 of the supplementary appendix there’s a list of the drug treatments that varied between the 2 groups. The two groups were treated differently
UPDATE – there’s been some discussion of the technique of ‘ordinal analysis’ that was used in the trial and pushed by the authors as a way of seeing this as a positive trial. EM Nerd has written a great little piece on it if you want to know more


Pingback: Stroke thrombolysis and IST-3 – is it another false dawn? | the underneaths of EM
Pingback: The IST-3 trial « Neurology Journal
Pingback: Il peso dell'umanità - EM Pills