So this is follow-up to Why MetHb is a bad thing. If you want a review of the basics read it then come back.
[peekaboo_link name=”Why would you ever want to have methaemoglobinaemia?”]Why would you ever want to have methaemoglobinaemia?[/peekaboo_link]
[peekaboo_content name=”Why would you ever want to have methaemoglobinaemia?”]
- hydrogen sulfide toxicity
- cyanide toxicity – though not so much as I think we have much better things to do with cyanide toxicity but that’s a whole other post…
[/peekaboo_content]
[peekaboo_link name=”What is this hydrogen sulfide that you speak of”]What is this hydrogen sulfide that you speak of[/peekaboo_link]
[peekaboo_content name=”What is this hydrogen sulfide that you speak of”]

- it’s what makes up that rotten egg smell when you go to thermal pools (as you do…) or when you err… pass wind… that obviously only applies to men as ladies do not break wind apparently. At least that’s what my wife tells me…
- you’re more likely to see it in an industrial setting where it has all kinds of uses
- there are some interesting case reports of people poisoned in thermal spas too
- the article from Japan in the references details a spate of suicides where household chemicals were used to produce the gas
[/peekaboo_content]
[peekaboo_link name=”Effects”]Effects[/peekaboo_link]
[peekaboo_content name=”Effects”]
Potentially this
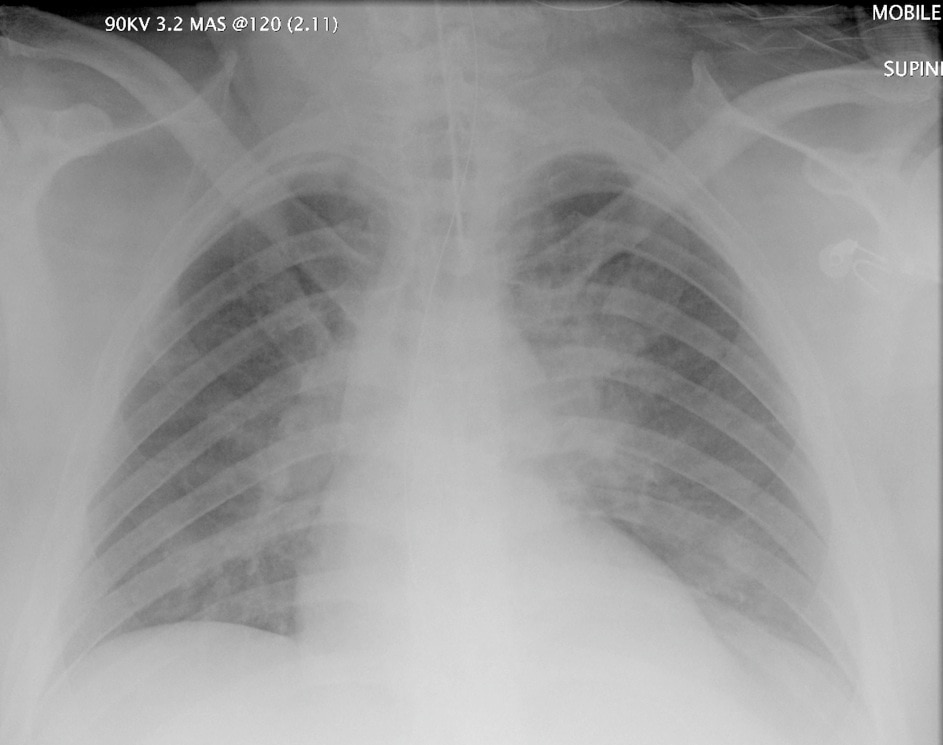
This was from a poor chap who got exposed in an industrial incident back when I worked in NZ. I looked after him in the ICU after the smart ED docs had done all the fancy interesting stuff I’d never heard of before…
Significant exposure means:
- “knockdown” meaning virtually instant LOC and loss of breathing
- irritation of eyes and lungs with bronchospasm
- inhibits cellular respiration in a very similar manner to cyanide (forms a bond with ferric (3+) Fe in mitochondrial cytochrome oxidase)
[/peekaboo_content]
[peekaboo_link name=”How do I know if a patient has been exposed”]How do I know if a patient has been exposed[/peekaboo_link]
[peekaboo_content name=”How do I know if a patient has been exposed”]
- hopefully someone will tell you (ambulance crew etc…) though we all know that doesn’t happen often enough
- they smell. No really they smell bad. Incidentally if the concentration is high enough the olfactary nerve stops working, so if you can’t smell it you’re too close to the patient and your eyes are about to start watering
- copper coins in the patients pockets should be discoloured
- the usual signs of cytotoxic hypoxia (sick sick patient with normal oxygenation but no utilisation in the tissues; high lactate; high ScvO2)
[/peekaboo_content]
[peekaboo_link name=”Management”]Management[/peekaboo_link]
[peekaboo_content name=”Management”]
- supportive care yada yada yada…
- personal protective malarkey probably matters here
- induce a metHb with either amyl nitrate or sodium nitrite
[/peekaboo_content]
[peekaboo_link name=”If I’m going to intentionally poison someone then I think I should know why?…”]If I’m going to intentionally poison someone then I think I should know why?…[/peekaboo_link]
[peekaboo_content name=”If I’m going to intentionally poison someone then I think I should know why?…”]
- MetHb acts as a scavenger; hydrogen sulfide has greater affinity for metHb than cytochrome oxidase (remember metHb has a ferric (Fe3+) part just like cytochrome oxidase) and therefore lets your mitochondria go back to metabolising like they’re meant to
- In simpler terms the hydrogen sulfide will jump ship from the mitochondria to the MetHb and therefore restore normal(ish) metabolism
[/peekaboo_content]
[peekaboo_link name=”Target MetHb?”]Target MetHb?[/peekaboo_link]
[peekaboo_content name=”Target MetHb?”]
- the idea is to induce somewhere between a 20-30% MetHb – in other words enough to clear out some of the H2S but not enough to kill an already very sick patient
- Medscape and UpTodate suggest 10ml of 3% sodium nitrite (or 300mg) based on when it was used in cyanide poisoing. There’s never any real evidence for this type of thing
- if you’re thinking this through then you’ll be aware that in someone with an Hb of 7, a 20% MetHb won’t be tolerated as well as someone with an Hb of 15
You may well never see this in your career but if you do see a hydrogen sulfide poisoning then at least remember this:
- similar to cyanide poisoning
- can be treated by inducing metHb
- use sodium nitrite
[/peekaboo_content]
References:
- UpToDate.com (subscription required)
- Medscape.com
- The ever helpful but occasionally inaccurate Wikipedia
- Morii et al.: Japanese experience of hydrogen sulfide: the suicide craze in 2008. Journal of Occupational Medicine and Toxicology 2010 PMCID 20920221

Pingback: Hardly a nice Pinot Noir but… | Emergency Medicine Ireland