I have been speaking at EuSEM for about 5 years now and it’s lovely to finally be back in person.
This is a summary of the talk I gave at the EuSEM 2021 conference in Lisbon. I’ll add a screencast in due course.

The crashing asthmatic has been a fairly standard talk at EM conferences for years. I’ve listened to a lot of excellent talks on it It’s something we spend a fair amount of time on as it’s a truly terrifying presentation for both patient and providers. But it is not common. At least I can say with confidence it is not common in my practice. I work in a fairly well developed health care system where everyone has access to primary care. Most people’s asthma is well controlled. There are always the small number of patients recalcitrant to treatment or non compliant but they are rare. As a result the truly crashing asthmatic has become less common over the 20 or so years I’ve been practicing.
I mention that as a caveat to this talk because the near-fatal, crashing asthmatic is not a common presentation. You will be able to manage the vast majority of severe asthma with the use of evidence based guidelines published by the major societies. The treatment in those scenarios is relatively straightforward
This talk is going to focus on the areas that off the map, the areas that lie off the map of the evidence based guidelines. This is a land that is largely evidence free. Decisions we make here are usually based on physiology and experience and all kinds of subjective preferences of beliefs. Unfortunately in the land that evidence forgot we find that the strength of the opinion on any given topic is inversely proportional to the level of evidence available to support that opinion. Discussing the crashing asthmatic can be a little like discussing airway management on twitter. Lots of very strong opinions without very much evidence. As a result you’re going to get a talk that has lots of pros and cons, on the one hand this, on the other hand that… especially when it comes to the drug treatments.
First off we need to make sure we’re treating the right diagnosis. There are a few presentations that we can mis label as asthma that aren’t.
The young female coming in by ambulance with a loud audible wheeze. Oxygen sats are fine but she has huge work of breathing and has told the crew she has been intubated 3 times for this. You can hear the wheeze across the room. If anything she worsens as you given more and more beta agonists and eventually she gets intubated. You wait for the crash post intubation but it never happens and she’s easy to ventilate and gets extubated the next day. This patient never had asthma. This patient has paradoxical vocal cord motion or vocal cord dysfunction. If you’d paid more attention to the pattern of the “wheeze” you would have noticed it was actually stridor. I have seen as many of these patients intubated in the ICU as I have true asthmatics.
The patient coming in with severe wheeze and hypotension and diarrhea and a history of asthma. You start treating for severe asthma on a background of severe gastro but you’ve missed the diagnosis completely. This is anaphylaxis. And while some of the treatments cross over, it is critical that this patient gets adrenaline and if you don’t consider it then they’re not going to do well
The older patient with a label of asthma who presents wheezy with hypercapnia but alert. The high CO2 makes you label this as a near fatal asthma attack and you launch in with your rescue treatments and set up for intubation. In fact this patient actually has mostly COPD and really just needs some NIV.
Let’s assume you’ve actually got the diagnosis right.
- For the basics we need
Beta agonists – people worry about the dose here but in reality there needs to be enough salbutamol in the nebuliser that it is running continuously. Every 5 minutes or so just top it up with some more. - Ipratropium. This sometimes gets forgotten, you only need two or three doses, don’t go crazy but get it in there early
- Steroids – will not work immediately but there is a clear benefit of steroids in acute asthma. For 95% of patients you can just give these orally but for the tiny proportion that are truly crashing just give them some IV be it hydrocortisone or dexamethasone or methylpred or whatever you normally use.
At this stage we’re largely talking about IV treatments. But why IV? This is a respiratory disease, affecting the large airways and we have a great route for administration be it inhaled or nebulised. Why would we give some systemically? Remember at this stage you’re treating someone with an almost silent chest. The person with loud expiratory wheeze on auscultation is really reassuring – they’re moving lots of air. When you listen and you hear very little then you start to worry. So for those with a silent chest it’s really unclear if our inhaled treatment are actually getting to the site of action, hence why we reach for the IVs
Magnesium has become almost a brain stem level reflex for the acute severe asthamtic patient. It is cheap and easy to give and rarely causes significant side effects. It’s evidence base is not stellar but remember we are approaching the edge of our asthma map here. The most recent cochrane on this is 2014
“This review provides evidence that a single infusion of 1.2 g or 2 g IV MgSO4 over 15 to 30 minutes reduces hospital admissions and improves lung function in adults with acute asthma who have not responded sufficiently to oxygen, nebulised short‐acting beta2‐agonists and IV corticosteroids”
Following magnesium the next drug I typically reach for is IV salbutamol. This does not seem to widely available in other countries so i’d be interested to hear other people’s experience. Physiologically it makes great sense. We know beta 2 stimulation of the smooth muscle is key which is why inhaled salbutamol works so well. Once they’re severe enough to not be able to get the inhaled drug to the site of action it makes perfect sense to give it IV.
There’s not much evidence with cochrane only finding 3 trials back in 2012 and concluding insufficient evidence. I’ve looked again and not found anything new apart from retrospective studies.
I did find this lovely review by Dr Tobin from Australia which made an interesting argument about the adverse effects of the metabolic load of IV salbutamol. We all know that when you stimulate beta agonists you get a concurrent rise in lactate levels. Be it adrenaline or salbutamol you will predictably see a bump in the lactate. That lactate contributes a metabolic load; an anion gap that the body will compensate for with increased minute volume. The crashing asthmatic is already struggling to do just that and raising the lactate might just be the thing that tips the patient over the edge.
This doesn’t stop me using IV salbutamol but it is something I keep in the back of my head now
The obvious alternative to IV salbutamol is of course adrenaline. We have been using it for years, it’s good enough to be produced by our own adrenal glands and it was the treatment of choice before inhaled salbutamol came along. I have used this in a push does fashion in the early stages of a creasing asthmatic while we are getting infusions running. Typically we’re talking 5-10mcg here. It comes with all the side effects that we see with salbutamol so the metabolic load argument applies here also. The other major concern is the archaic and confusing way that adrenaline comes in with homeopathic sounding formulations of 1:1000 and and 1:10000. There is a real opportunity for drug error here so be careful.
NIV I think is a great option. The hope is to increase the functional residual capacity and reduce work of breathing. In the early stages of acute asthma the patient will have lots of energy and ATP floating around and a diaphragm ready to run a race. 45 minutes into the attack the diaphragm is fading and the tidal volumes are falling. NIV can help augment the respiratory muscles and keep the minute volume high enough to clear the CO2 and avoid intubation. Remember that auto-PEEP; gas trapping, can occur in spontaneously breathing patients. At the end of inspiration there is still a +ve pressure at the alveoli due to the air trapped in the large airways. In order to initiate the next breath they have to generate a sufficient negative pressure with the muscles and diaphragm to overcome that auto-PEEP.
NIV can help with all of that.
Cochrane has not looked at this since 2012 when surprise, surprise it found insufficient evidence. There have been a few trials since, all suggesting, benefit of NIV. It’s hard to know exactly when to start this but ideally you’d want to try it while the patient is still cooperative. They will need coaching, and I typically start at almost zero settings with high flows just to get them used to the mask then slowly titrate up the pressures but typically keeping both IPAP and EPAP in the single digit range.
This is the stage I would introduce NIV, after mag, after IV salbutamol and while the patient is ideally still cooperative and able to engage. If the patient is too agitated to tolerate NIV then I would still give it a go but we’re going to need some sedation.
Ketamine is a greatly loved drug in EM for many reasons and it probably has a bronchodilatory role in asthma. Again this is an off the EBM map type of intervention. Again cochrane finds little data and little support but that is hardly a surprise and would not necessarily put me off. The only more recent RCT i could find was in kids that compared aminophylline with ketamine and concluded that both were equally efficacious. Given that aminophylline was the comparator it would also be fair to say that both were equally useless.
The dosing is also hard to clarify. The reported literature is mostly observational or case reports with people giving whatever they wanted. In general, it trended toward dissociative doses, ie 1-1.5mg/kg rather than the sub dissociative pain doses that we might be used to. Honestly though i think you just need to pick a dose and go with it. At this stage I am preparing for an intubation so I am likely to go with a dissociative dose. I would not do that unless I am literally ready to go for intubation as this is the critical time when the patient may unravel and arrest on you so be prepared. As a correlate to that I would not use benzos. Too many times a small dose of benzos has led to the decompensation of a respiratory failure patient. These are not great options.
The real controversy starts in the use of sedation to facilitate NIV. For me this is where the dissociative dose of ketamine comes in. So I’m using the ketamine for 2 reasons. 1) as a bronchodilator and 2) as a means to facilitate NIV. For the cooperative patients I won’t need this. At this stage we are ready to intubate with ketamine and NIV as the last ditch attempt to maintain oxygenation and ventilation.
The next obvious question is when to intubate
Wait longer than you normally would
Wait a little bit more
But not too long
Typically in respiratory failure the combo of acidosis, hypercapnoea, tiring and falling GCS would all be sensible indications to intubate. But given the issues with delivering safe positive pressure ventilation I will often let them go a fair amount further before tubing tolerating a lower GCS and a lower pH in the hope of reversing the bronchospasm. It goes without saying that you will not leave the bedside in the 1-2 hrs it will take to progress through these treatments and decisions.
Let’s say we’ve reached the threshold and we know that in the next 5-10 minutes the combination of poor GCS, poor cough, acidosis, hypercapnoea and reduced respiratory effort is going to result in a cardiac arrest. We know we have to intubate.
I would use a dose of ketamine and a paralytic and in reality the tube will almost always be easy. I would use a big tube, for reduced airway reasons and additionally to facilitate bronchoscopy for clearance of mucous plugs. I can fit a bronch down a 7 but i can’t ventilate whereas if you put an 8.5 in then i can ventilate and bronch.
Typically in respiratory failure the combo of acidosis, hypercapnoea, tiring and falling GCS would all be sensible indications to intubate. But given the issues with delivering safe positive pressure ventilation I will often let them go a fair amount further before tubing tolerating a lower GCS and a lower pH in the hope of reversing the bronchospasm. It goes without saying that you will not leave the bedside in the 1-2 hrs it will take to progress through these treatments and decisions.
Let’s say we’ve reached the threshold and we know that in the next 5-10 minutes the combination of poor GCS, poor cough, acidosis, hypercapnoea and reduced respiratory effort is going to result in a cardiac arrest. We know we have to intubate.
I would use a dose of ketamine and a paralytic and in reality the tube will almost always be easy. I would use a big tube, for reduced airway reasons and additionally to facilitate bronchoscopy for clearance of mucous plugs. I can fit a bronch down a 7 but i can’t ventilate whereas if you put an 8.5 in then i can ventilate and bronch.
The main issue comes when you connect the vent.
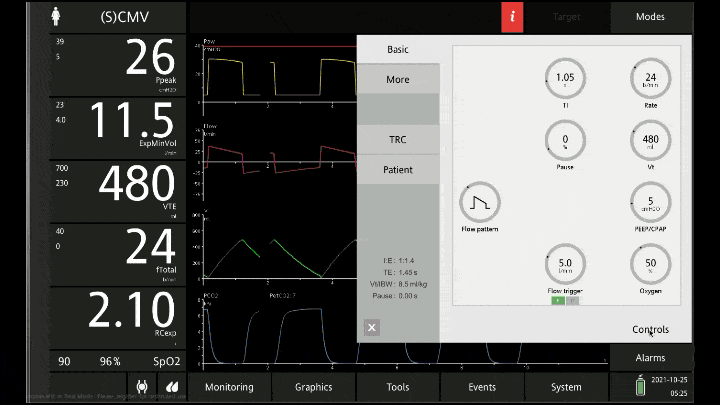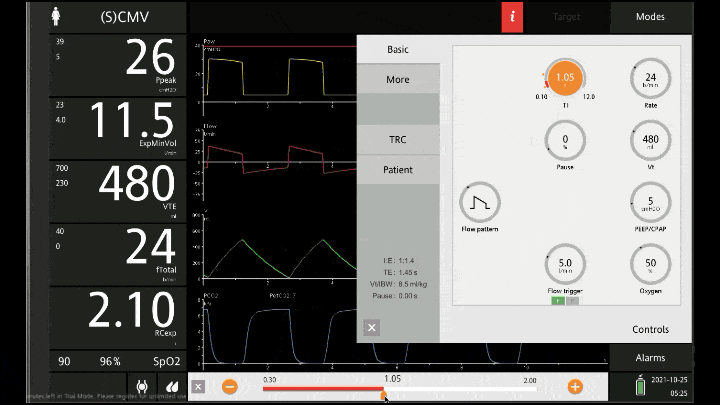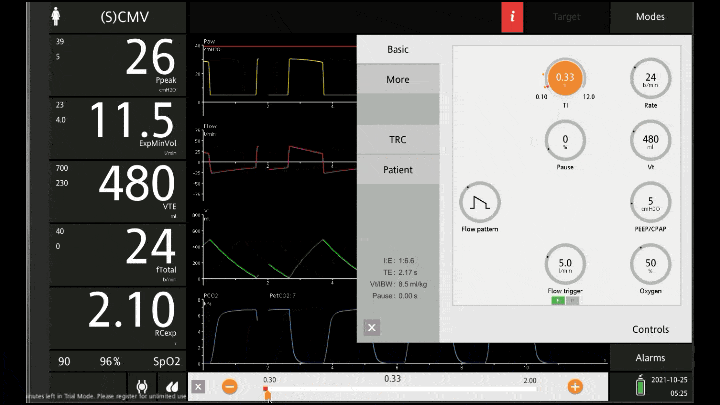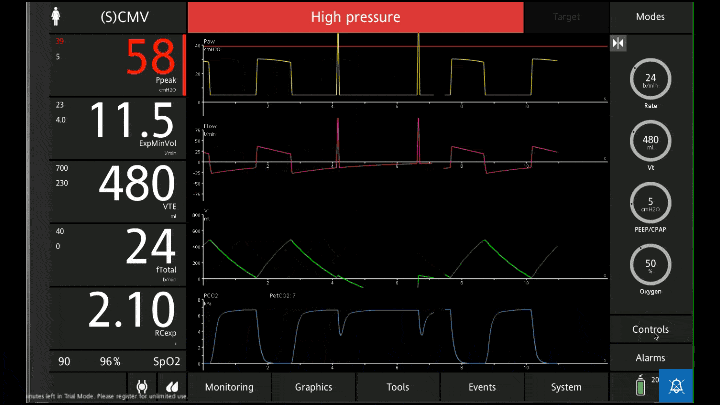
Air will go into the lungs fairly easily, the vent will complain about high airway pressures but will do what it’s told. The problem is that for every 400mls we put in only 350 come out before the next 400mls go in. As a result with each breath we’re adding 50 mls or more to the volume of the lungs and we end up in a situation of dynamic hyperinflation or gas trapping.
[A note on these vent waveforms. These were created with the Hamilton C6 simulator. The closest simulation i could find was COPD so I know this doesn;t look like classic ashtma but hopefully it gets the point across. We have servos in our place so no COI to declare!]
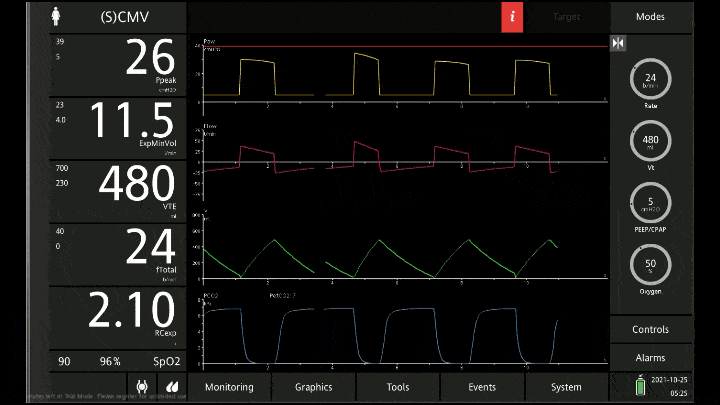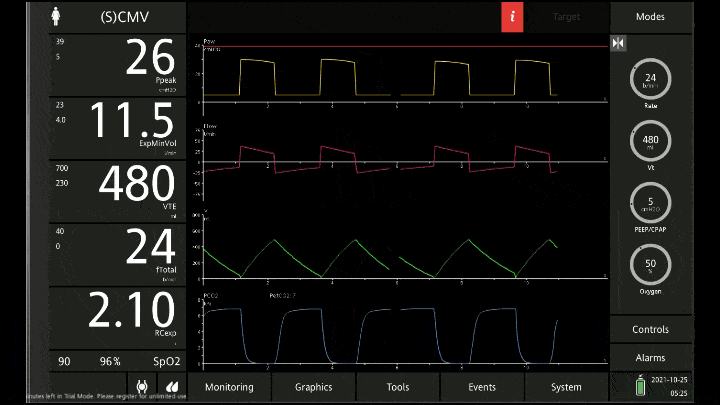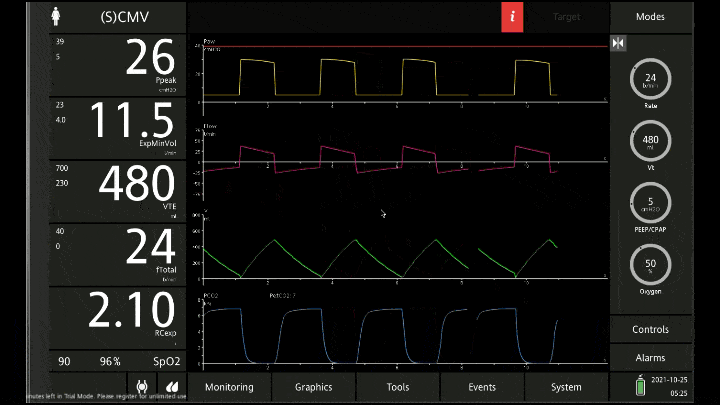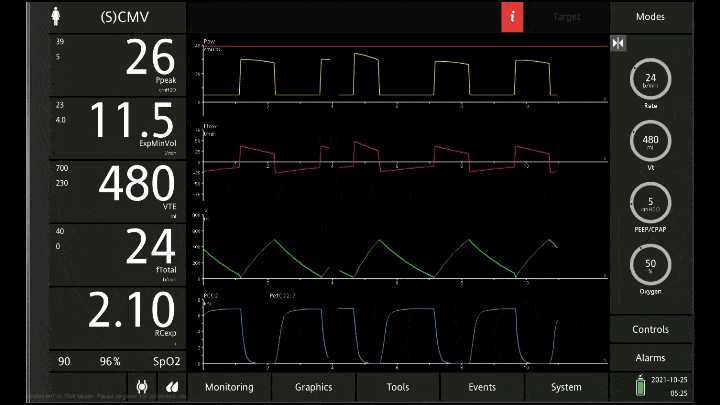
There are 3 major consequences to this
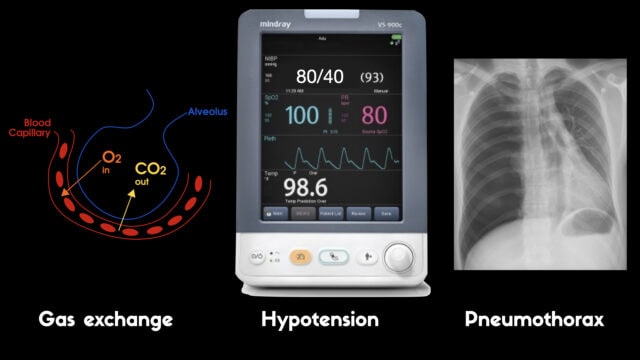
1) Eventually the vent will not be able to move gas and CO2 will rise, pH will fall and eventually hypoxia will develop as the body burns through whatever oxygen was in the alveoli before fresh gas stopped arriving
2) The high pressure in the thorax will impede venous return like a clamp being slowly tightened over the SVC and IVC inlet. Less and less blood returns to the heart and the pressure drops and the patient loses a pulse
3) The high pressure in the lungs ruptures the parenchyma and gas under pressure fills the pleural space resulting in tension pneumothorax and subcutaneous emphysema and inevitable cardiovascular collapse and arrest.
To detect these issues you need to be able to read a few things on the vent waveform. You need to be able to read the plateau pressure and you need to be able to read the flow waveform.
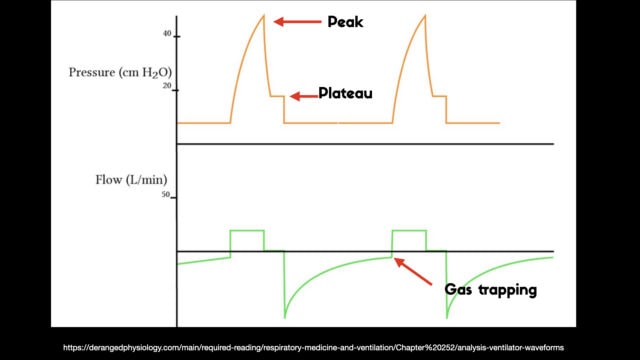
The flow waveform tells us if expiration is complete before the next breath is complete. If the flow waveform returns to baseline before the next breath then everything is good. If the next breath begins before that waveform reaches zero then we know we’ve got extra gas accumulating in the thorax with each breath.
The plateau pressure is the pressure in the circuit when the breath is held at end inspiration. There is no flow here, just the pressure in the circuit at the very end of inspiration. This is the pressure that the alveoli are feeling. Ideally this would be less than 20 but in this type of situation I would live with less than 35-40. You’ll notice that the peak pressure is often much, much higher. This is the highest pressure recorded while the ventilator is squeezing air into the lungs and this is reflective of the pressure felt by the large airways. This can be effectively disregarded. The flow waveform tells us if expiration is complete before the next breath is complete. If the flow waveform returns to baseline before the next breath then everything is good. If the next breath begins before that waveform reaches zero then we know we’ve got extra gas accumulating in the thorax with each breath.
You’ll notice that the peak pressure is often much, much higher. This is the highest pressure recorded while the ventilator is squeezing air into the lungs and this is reflective of the pressure felt by the large airways. This can be effectively disregarded.
The flow waveform tells us if expiration is complete before the next breath is complete. If the flow waveform returns to baseline before the next breath then everything is good. If the next breath begins before that waveform reaches zero then we know we’ve got extra gas accumulating in the thorax with each breath.
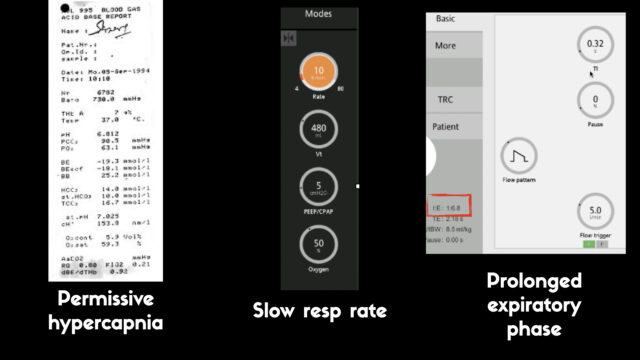
We can make ventilation safer by doing a few things:
- Accepting a hypercapnoeic acidosis
- Slowing the resp rate
- Prolonging the expiratory phase (but it’s vital to note that prolonging the expiratory phase is only any use if you have already lowered the resp rate.)
If you were to see a pH and PCO2 of 10 kpa immediately after intubation the reaction will be turn up the minute volume either by increasing the RR or increasing the Vt but this is the wrong move in our asthmatic. I’d be doing high 5s if I got that kind of gas post intubation.
It is unclear what a safe pH is for this type of patient we are usually pretty liberal about things given that the risks of uncontrolled dynamic hyperinflation probably outweigh the risks associated with respiratory acidosis. Typical recommendations come in the 7.15-7.2 range but ultimately you do whatever you have to to oxygenate the patient while not causing them to explode. If the patient was tolerating it I would be tolerating pH below 7.1 if needed.
The main reason for the lower resp rate is to allow more time for expiration. The respiratory cycle is a zero sum game, there is no way to simultaneously have a high minute volume and extra time to expire. You have to fit it all into the one inspiration and expiration. If you set a RR of 20 with an I:E of 1:6 you’re only allowing the vent 0.3 of a second to complete inspiration before it cycles into expiration which is usually not enough time for the air to squeeze in all the gas. The vent alarms and the tidal volume isn’t delivered. But when you set the RR at 8 or 10 it allows you to set I:E ratios of 1:5 or longer.
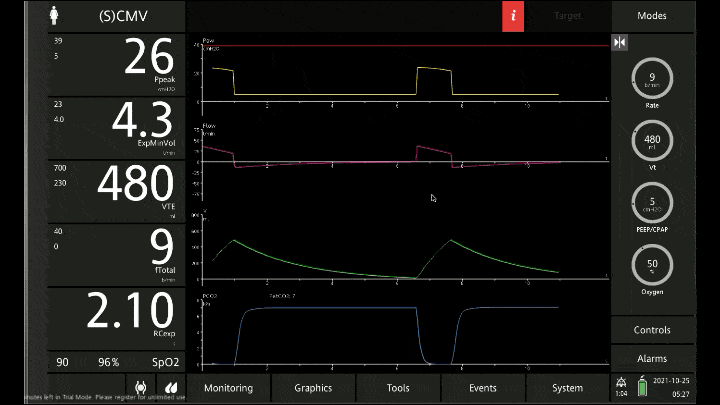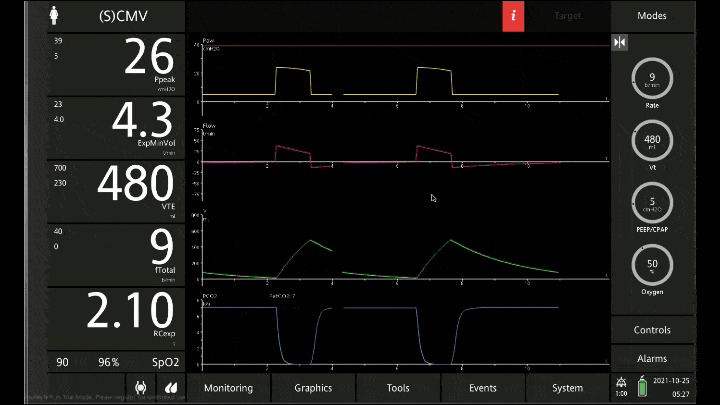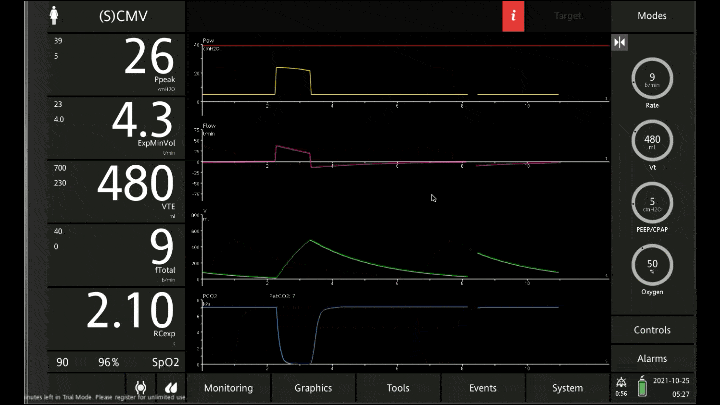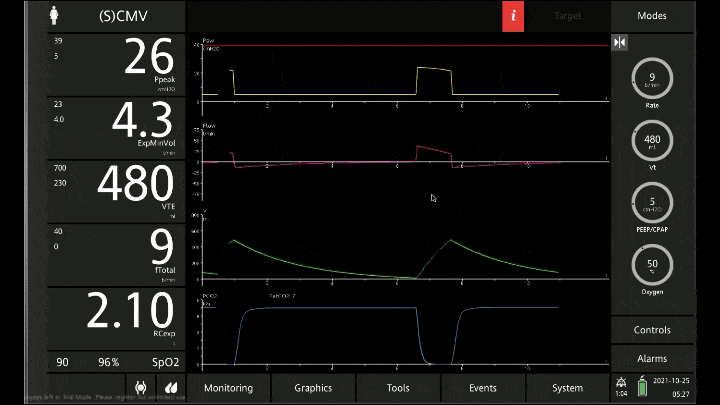
Now with the lower rate we can see there is enough time for inspiration to occur and enough time for the flow to return to zero to ensure there is no gas trapping.
Once the tube I would advise keeping them paralysed, which again goes against all usual good ICU management where we want them to start breathing on their own. I want them paralysed for 2 reasons, firstly i don’t want them breathing up against my very low RR that I have sent. It is almost impossible to maintain permissive hypercapnoea in a patient who is not paralysed – the brainstem reflex to breath is just too powerful. Secondly paralysis is a useful adjunct in reducing the amount of CO2 produced. CO2 is a by product of metabolism be that from muscle contraction or cerebral blood flow so sedation and paralysis reduces the burden of CO2 that needs cleared.
Lets say you’ve got the vent wrong and 5 mins into ventilation the BP and sats are dropping. The first thing to do is disconnect the patient from the vent. You may even need to physically squeeze the chest to help get some fo the air out. If dynamic hyperfinflation was the problem this would have fixed it.
If the patient is still crashing then get some fluid going and think about pneumothorax. US and CXR can be helpful and decompressing the pleura with a finger thoracostomy or a chest drain will fix the problem and answer the question.
No talk like this would be complete without mentioning ECMO. ECMO has become the answer to everything. I work in an ECMO intensive care unit and I think my take home from moving from the ED to ICU is that ECMO is often recommended in talks like these and rarely done. Mostly this is because ECMO is actually not needed, even for the sickest of ICU level asthma patients. It’s also logistically hard to do, if you need to call a team in to do VV ECMO then the bronchospasm is often waning by the time they arrive. Where it really comes in is when you have complications from ventilation. If you’ve blown bilateral pneumothoraces and you’ve got a bronchopleural fistula then you might need it, or you’re in a situation where the pH is so low that the patient has maybe already arrested then that’s when we’re thinking about it.
So in summary. This kind of asthma is rare, and getting rarer by the year. As a result there is very little evidence to guide us and most of this is physiological reasoning. It is really important to know that ventilation is dangerous. We usually view intubation as our get of jail card in respiratory failure. We know that if all else fails we can just tube them and fix them but in asthma the problems are only really beginning once the tube goes in. It is really important to know that ventilation is dangerous. We usually view intubation as our get of jail card in respiratory failure. We know that if all else fails we can just tube them and fix them but in asthma the problems are only really beginning once the tube goes in.
A little kudos to some of the FOAM resources used in preparation. Josh Farkas and the IBCC have an excellent chapter and podcast covering this. Weingart from EMCrit has an almost seminal podcast way back from 2013 the is compulsory listening and more recently Haney Mallemat from Critical Care Now has an excellent video from SMACC covering the Crashing asthmatic. Reuben Strayer who you’ll hear from shortly, has also done some nice FOAM work surrounding this.
References:
ICU fundamentals on EMRAP with Sara Crager (Paywall)
A guest post on opioids on EMCRIT
Some Cochrane

Really comprehensive Andy! Thanks
Thanks for all the great lectures!!! They are an invaluable resource!
Would you worry about using an atracurium infusion in the bronchospastic patient, or is this a theoretical consideration?
Thank you very much
no, the risk with things like rocuronium is already really low and with atracurium even lower as far as i can tell.